Debrief -> Science Chemistry MCQ
-
Discuss here

-

-

-
opps i rmb now
-
My suggested answer:
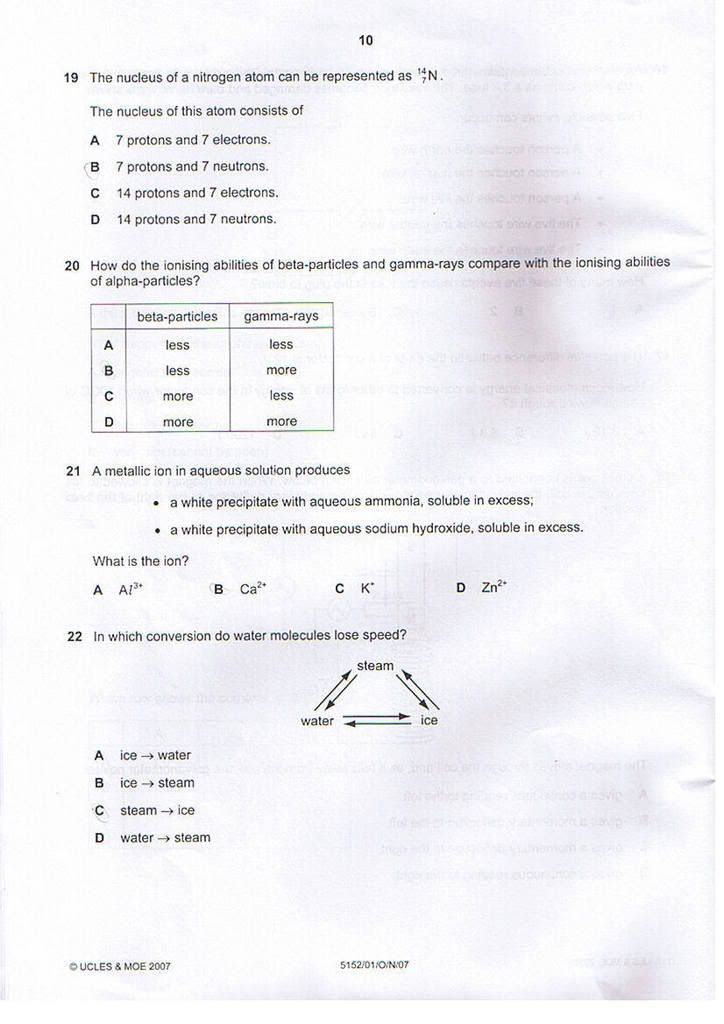
Q21) D
Q22) C
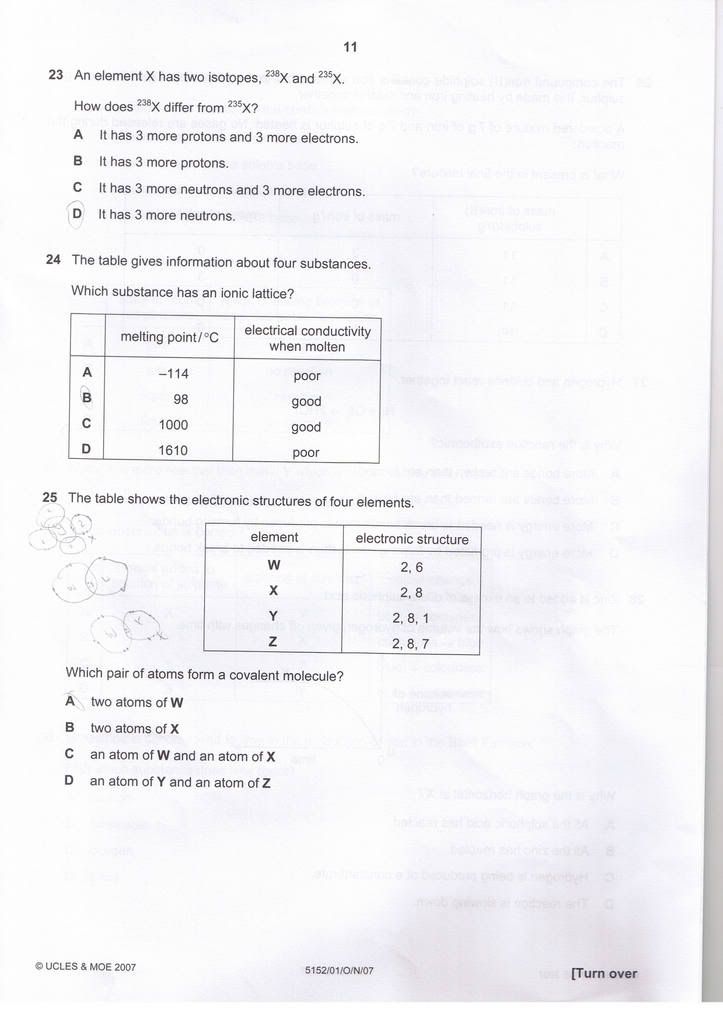
Q23) D
Q24) C
Q25) A
Q26) B
Out of the 20 chemistry question, the question I like most will be this. On the surface it looked simple, but it's testing Limiting reactant, and calculation of excess reactant.
First step is to determine the molecular formula. After you've done with your calculation, you'll get FeS.
Then next is to find out which is the the limiting reactant. After the calculation, you'll know that Fe is the limiting reactant, and of course S is in excess.
No. of mol of FeS/ No. of mol of Fe = 1/1 = 1
No. of mol of FeS = 1 x 0.125 = 0.125 mol
Mass of FeS = 0.125 x (56 + 32) = 11g
Final step is to fine out the mass of excess reactant(S).
As calculated earlier(not shown here), take the no. of mole of S minus the no. of moles of Fe. Which is 0.219 - 0.125 = 0.094 moles of S in excess
Then 0.094 x 32(Ar of S) = 3.
Answer: B
Q27) D
Q28 ) B
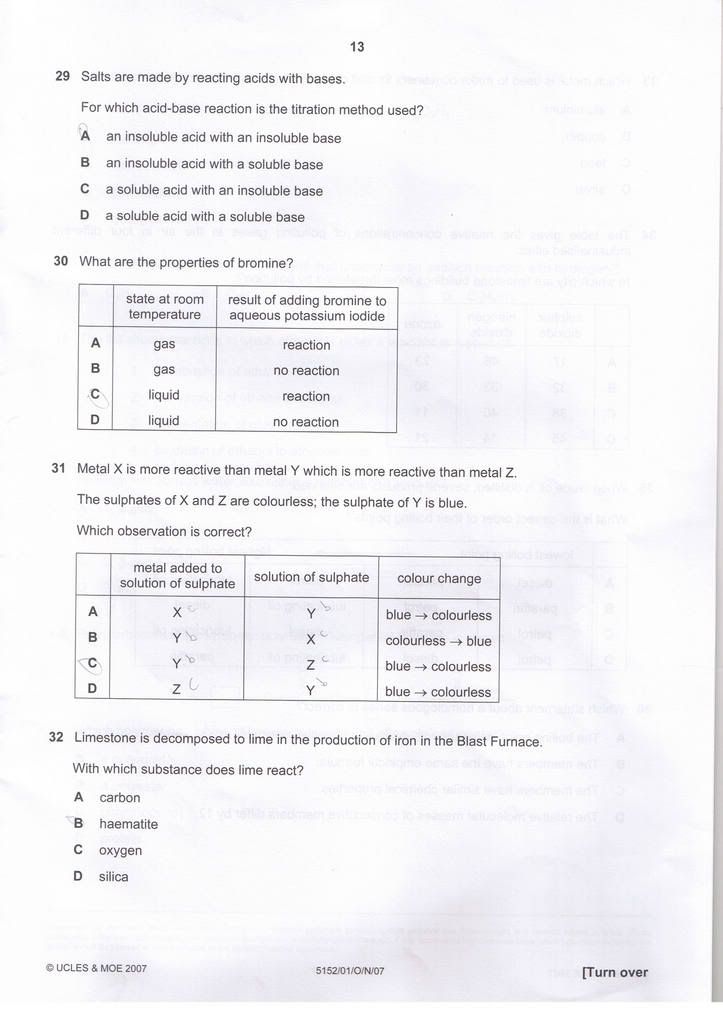
Q29) D
Q30) C
Q31) A
Q32) D
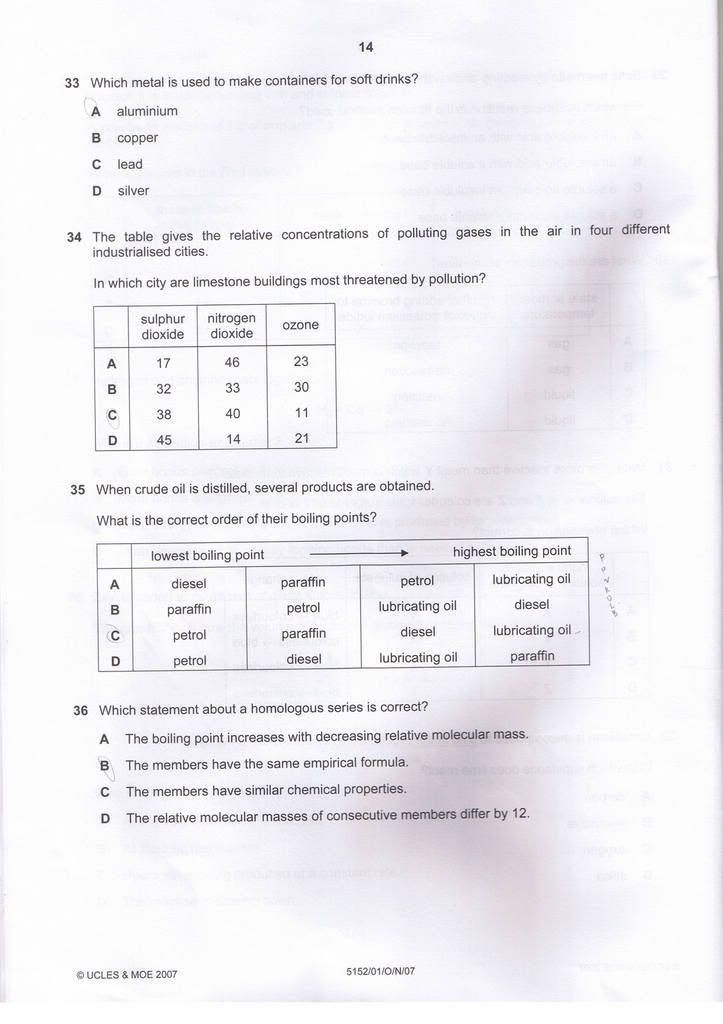
Q33) A
Q34) C
Q35) C
Q36) C
Q37) A
Q38 ) B
Q39) A
Q40) B
Edited for Q34, thanks kester for pointing out my mistake.
-
Oh,i dont wanna check my ans.Scared of finding out how bad i done

-
Chemistry ah..i see i sian ah
 Next monday i die liao
Next monday i die liao -
that person who posted the question papaer got alot wrong leh.

-
This O Level Paper belongs to another person.Originally posted by Y_Shun:that person who posted the question papaer got alot wrong leh.
-
although i dont take this paper
but the FeS question
I think that first u get 7+7 = 14g
14g - 11g = 3g
3g is the mass of sulphur
Iron is limiting so nothing left
so answer is 11g Fes 0 Fe 3g S -
Assuming Darkness_hacker99 answers are correct, which I'l ltrust him on this one.
Chemistry MCQ : 18/20.
Not too bad actually.
Fermentation doesn't involve water. How silly of me =.="
Regarding Q34, Doesn't both sulphur dioxide and nitrogen oxide affect limestone ?
My ans is C. -
You are right.Originally posted by kester:Regarding Q34, Doesn't both sulphur dioxide and nitrogen oxide affect limestone ?
My ans is C.
Both SO2 and NOx does plays a part in Acid rain. I admit my mistake.
Thanks for pointing out

-
may i ask
why is qns 24 C?
why is qns 40 B?
oh, i tink i got 3 wrong.
-
Originally posted by PwnYou:may i ask
why is qns 24 C?
why is qns 40 B?
oh, i tink i got 3 wrong.

The answer is C.
The ions in ionic compound are arranged in a lattice form, that why it's called ionic lattice.
Property of Ionic compound:
1) When molten, it conducts electricity due to moving electrons.
2) have high melting points.
The answer is B.
The word 'poly' means many. Comparing the linkage which I have posted(below), there is a different between 'Amide' and 'Ester' Linkage.
If you look can see... Polyester seems to be the most similar out of the other options.