Stuck with physics gas questions
-
Originally posted by eagle:
C19 P38
temperature the same, kinetic energy of molecules should be the same
thus, (0.5)(m1)(v1) = (0.5)(m2)(v2)
but v2 = 2v1,
m1/m2 = v2/v1 = 2
Not confirmed though....
ahh i have a hint:x
You do not need all the information given. Did you write the equations for average speed and rms speed? How is molar mass related to molecular mass?
-
that's O level chemistry I think...
molar mass = molecular mass * mole number
-
Originally posted by eagle:
C19 P38
temperature the same, kinetic energy of molecules should be the same
thus, (0.5)(m1)(v1) = (0.5)(m2)(v2)
but v2 = 2v1,
m1/m2 = v2/v1 = 2
Not confirmed though....
Oops... Careless with the above formula... Should be 1/2 m v^2
(0.5)(m1)(v1)^2 = (0.5)(m2)(v2)^2
but v2 = 2v1,
m1/m2 = (v2/v1)^2 = 4
-
i think that's related to the value "R"
ps: 4 isnt the ans
-
With this formula
p = (nMv^2)/3VpV/n = Mv^2 / 3
this is a constant because R and T are constants here
so,
m1v1^2 / 3 = m2v2^2 / 3
Ratio of m1/m2 is still 4 leh...
I think different p means different V or n only... The Vrms is still heavily dependent on temperature.
-
arrr....
use Vavg and Vrms equations and rearrange to get m1/m2 ratio
thats how someone else got it >__< but how :O
-
OH!!!!!! SORRY.... didn't see it is Vavg!
According to your equations,
Vrms2 = Vavg * sqrt (3 * pi / 8) = 2.171 Vrms1
So should be 2.171^2 = 4.71?
-
4.71 is the correct answer! XD
-
apologize for being so careless :(
-
C19 P43
a) 0J as volume didn't change
b)

change in T = 16.3
N = 2.42 mol
f = degree of freedom = 3 for monoatomic gas
k = boltzmann constantchange in thermal energy = 491.7 J => not confirmed
c)

change in internal energy = a) + b) = 491.7J
d) should be 491.7J / (2.42 * 1mol) = 3.37e-22 J
-
thanks, that's the correct answer ^^
-
C19 P61
a)
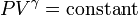
1.8 * 176^y = 5.51 * 79.2^y
2.2222^y = 3.061111
y = ln(3.061111) / ln(2.222222) = 1.4=> diatomic gas
b)
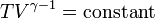
so,
310 * 176^1.4 = T * 79.2^1.4
T = 948.1Kc) PV = nRT
so n = PV/RT
p = 1.80atm, 1 atm = 101325 Pa
V = 176L = 176000cm3 = 0.176 m3
R = 8.314 J·K−1mol-1
T = 310Kthus, n = 6.92 mol
-
b and c cant get correct...hint is to apply the ideal gas law. anyways cant reply tonight..
-
Originally posted by eagle:
C19 P61
a)
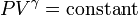
1.8 * 176^y = 5.51 * 79.2^y
2.2222^y = 3.061111
y = ln(3.061111) / ln(2.222222) = 1.4=> diatomic gas
b)
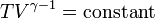
so,
310 * 176^1.4 = T * 79.2^1.4
T = 948.1Kc) PV = nRT
so n = PV/RT
p = 1.80atm, 1 atm = 101325 Pa
V = 176L = 176000cm3 = 0.176 m3
R = 8.314 J·K−1mol-1
T = 310Kthus, n = 6.92 mol
Part b careless.... Equation is still correct... Anyway you should also help check for careless mistake mah... Not just take the values and use them without trying to understand how the equation was used...
The power should be 0.4, not 1.4
310 * 176^0.4 = T * 79.2^0.4
T = 426.65KPart c is already ideal gas equation. Maybe you could help check if the calculations are wrong...
-
hmm im not sure for part c... i keep hitting a wall... will redo tonight.. still soo tired from last night
-






