BedokFunland JC's A Level H2 Chemistry Qns (Part 2)
-
AlphaEternala asked, "I don't like my school notes. How should I best answer H2 Chemistry questions in the A levels?"
No, there is no secret shortcut way to determine the perfect way to answer. As it is, Singapore JC teachers all give their own different answers, not just across JCs, but even within a JC.
It's common knowledge that after sharing Prelim papers across JCs, school teachers will open criticize other JCs' pelim paper questions and given answers in the mark schemes, saying to their students, "Don't follow their mark scheme answers, you should instead follow the notes I gave you".
So you should still follow yourself, write what you're comfortable with, even if your school lecture notes say otherwise. A general rule of thumb to guide you (ie. to make sure you will score most, if not all, the marks for the question in the A levels), is to ensure your answer includes all the main points and keywords found in reliable A level publications (ie. *not* school lecture notes, but quality A level books from CS Toh, George Chong, Chan Kim Seng & Jeanne Tan, etc). Combine the answers or keywords from 3 of your personal favourite reliable sources, and write it in your own style that you can be satisfied with (instead of reluctantly and blindly memorizing your school lecture notes answer which you personally disagree with).
To further allay your fears, rest assured that Cambridge allows a reasonable degree of flexibility in the phrasing of the answers. For instance, to a certain question, eg. "Why do transition metals form coloured compounds?", all 3 books CS Toh, George Chong, Chan Kim Seng & Jeanne Tan, would phrase their answers very differently. But Cambridge would award full marks to all 3 of them.
Moral of the story : so go ahead and combine their answers and keywords into your own unique answer that you're personally happy with. *Make it your own*. That's the secret to mastering Chemistry. -
Originally posted by hoay:
The boiling point of CCl4 is higher than SiCl4. CCl4 has Vander waal forces as well as SiCl4 has but the b.p of SiCl4 is expected to be higher than CCl4 as the former has more spacious electron cloud. Can someone explain.
I actually posted the answer to this question on my BedokFunland JC website several years ago :
Due to the greater magnitude of electronegativity difference between Si and Cl, compared to C and Cl, there is a larger magnitude of partial negative charge on the Cl atoms in SiCl4 molecules (compared to CCl4 molecules), and consequently a greater extent of electrostatic van der Waals repulsions between SiCl4 molecules, and therefore SiCl4 has a lower boiling point, compared to CCl4. -
Originally posted by hoay:
The following is aquestion from CIE A-level (9701) Nov 2010
G is NH2(CH2)3NH2 is titrated with HCl(aq).
A 0.10 mol dm–3 solution of G has a pH of 11.3. When 30 cm3 of 0.10 mol dm–3 HCl is added to 10 cm3 of a 0.10 mol dm–3 solution of G, the final pH is 1.6.
Using the following axes, sketch the pH changes that occur during this addition of
HCl(aq). [I cannot show the axes here]
No other data such as pka values were given. Its was a 2 marks question.
Solution : I only knew the starting pH that is 11.3 and the end pH 1.6. There will be two humps as the base is diacidic. The end-point i have no idea. Please help.
-
Here is my BedokFunland JC answer to this 2010 Cambridge A level Exam Qn.
Since molarities of both acid and base are the same, the volumes of HCl required for 1st and 2nd equivalent points are 10cm3 and 20cm3.
Show a steeper and longer vertical portion for the 1st equivalence point, with slightly acidic pH, eg pH 6.
Show a less steep and shorter vertical portion for the 2nd equivalence point, with a strongly acidic pH. eg pH 2.
Rationale :
Although technically diprotic, but you can deduce that it in practice it is mostly monoprotic (ie. extremely small Kb2), because of 3 (inter-related) reasons :
1) Considering the unipositive conjugate acid, the unipositive formal charged N atom / unipositive BH+ ion, electrostatically repels the H+ / H3O+ cation. Hence kb2 would be extremely small.
2) Considering the unipositive conjugate acid, the unipositive formal charged N atom is electon-withdrawing by induction, making the lone pair on the other N atom (3 C atoms away) to be less available to accept protons. Hence kb2 would be extremely small.
3) Considering the dipositive conjugate acid, 2 positively formal charged N atoms with only 3 C atoms between them is highly destabilizing, due to inter-nuclei repulsions within the highly unstable high cationic charge density dipositive conjugate acid. The more unstable the conjugate acid, the weaker the conjugate base. Hence kb2 would be extremely small.
As such, regard 1,3-diaminopropane as mostly monoprotic, in which case you can (even without Kb values available) expect the 1st equivalence point to have a acidic pH (due to proton dissociation hydrolysis of the acidic ammonium salt).
Which is why we draw a steeper, longer vertical portion for the 1st equivalence point, and a less steep, shorter vertical portion for the 2nd equivalence point (since Kb2 will be extremely small).
As for the strongly acidic pH at the 2nd equivalence point, as explained earlier, Kb2 is extremely small, meaning Ka1 (ie. proton dissociation for the dipositive conjugate acid) is extremely large, meaning that the dipositive conjugate acid is a strong acid. Hence at 2nd equivalence point, the dipositive diammonium salt is strongly acidic and will have a pH almost as acidic as the final pH of 1.6 (ie. with 10cm3 excess HCl). A reasonable estimate would be approx pH 2.
Originally posted by hoay:Thank you for the detailed answer.
Is there any book you would recommend for these type of pian-staking problems?
If you're referring specifically to acid-base equilibria problems involving polyprotic / multiprotic acids & bases, there's plenty of free materials available on the internet :
https://www.google.com.sg/#q=polyprotic+acid+base+equilibrium+problems -
My Original BedokFunland JC H2 Chemistry Challenge Qn (January 2015)
Draw the structure of the major product for each electrophilic aromatic substitution reaction.
[ Note : in all 3 cases, assume only 1 electrophile is substituted onto the benzene ring (ie. mono-substitution), and there is only 1 single most major product (ie. ignore the minor products and give only 1 answer). Hint : You have to simultaneously consider *both* steric and electronic (including *both* inductive and resonance) effects to arrive at the *single* most major product.]
Case 1 : 4-nitro-benzaldehyde : meta-directing moderate deactivator vs meta-directing strong deactivator, who wins? Identify all relevant factors (sterics / induction / resonance).
Case 2 : trichloromethylbenzene : is this substituent activating or deactivating? Is it ortho/para or meta-directing? Identify all relevant factors (sterics / induction / resonance).
Case 3 : 3-methyl-nitrobenzene : ortho/para-directing weak activator vs meta-directing strong deactivator, who wins? Identify all relevant factors (sterics / induction / resonance).
Case 4 : 3-bromo-nitrobenzene : ortho/para-directing weak deactivator vs meta-directing strong deactivator, who wins? Identify all relevant factors (sterics / induction / resonance).
Case 5 : 2-chloro-5-bromo-nitrobenzene : a 3 cornered fight, who wins? What's the single most major product? Identify all relevant factors (sterics / induction / resonance).
Special Note : I will not reveal the answers here ; ask your own school teacher or private tutor for their answers, but regard their proposed answers critically with a pinch of salt and ask yourself if you're truly satisfied with their explanations, as I wrote these tricky BedokFunland JC questions myself, and many school teachers and private tutors may not get their anwers correct.
On a related note, check out the following Yahoo Q&As :
https://answers.yahoo.com/question/index?qid=20100430074722AAB69Ac
https://answers.yahoo.com/question/index?qid=20111228172010AAonefP
but be warned : both the chemical engineer and the organic chemist got their answers wrong. Can you / your school teacher / your private tutor figure out what's wrong with their answers, and what the correct answers are? -
The difference between Chemical Biology versus BioChemistry :
http://www.brandeis.edu/departments/chemistry/docs/Chemical%20Biology.pdf -
Originally posted by hoay:
The by-product of electrolysis of brine is sodium hydroxide. The reaction that is quoted is 2H2O + 2e - H2 + 2OH- .....which occur at cathode. The problem is that, surely at the cathode cations are discharged such as here H+ ions, should be discharged. No idea how water being a neutral species migrates to cathode ?? Please explain.
Within the H2O molecule, because O atoms are more electronegative than H atoms, hence the H atoms, having a partial positive charge, are electrostatically attracted to, and accept electrons from, the cathode. For every H2O molecule, 1 H atom accepts 1 electron, generating an OH- ion and a H atom, which readily combines with another H atom from the reduction of the next H2O molecule, thus generating H2 gas (with positive, thermodynamically favorable entropy change).
If you prefer, an alternative acceptable way to look at it, is that H+ ions (or H3O+ ions) from the auto-dissociation of H2O molecules, are electrostatically attracted to, and accept electrons from, the cathode, to be reduced to H atoms, which readily combines with the next H atom to generate H2 gas (again with positive, thermodynamically favorable entropy change), thereby causing the position of equilibrium of the auto-dissociation of water to shift to the RHS as predicted by Le Chatelier's principle, thus generating more OH- ions as a byproduct.
Both ways of looking at this are correct, use whichever you prefer, whichever helps you understand better, and Cambridge certainly won't ask for such explanatory details at A levels in any case. -
Originally posted by hoay:
Oh ! I missed this point. Thank you.
One more thing.........
If Cl- ions and Br- ions are present in the same solution with equal concentration then which is oxidized preferentially Cl- or Br- ?? E for Cl- is more postive (+1.36V) than Br- (+1.07V) then Cl- should discharged at anode but Br- is discharged in actual practice....any expalnation??
It is advisable to specify either "reduction potential" or "oxidation potential" when discussing feasibility of redox reactions.
The standard oxidation potential of 2Br- to Br2 is -1.07 V, which is more positive compared to the standard oxidation potential of 2Cl- to Cl2, which is -1.36 V.
Hence, assuming standard or equal molarities, the oxidation of Br- ions to Br2 is more feasible compared to the oxidation of Cl- ions to Cl2 at the anode. -
Originally posted by hoay:
Nitrate salts are not used as electrolyte insted of sulfates such as copper(II) nitrate. Is it due to the NO3- (+0.94V) having lower reduction potential than sulfate ion (+2.01V) so they may be discharged at anode eleasing toxic NO2 gas...?
At the anode, oxidation occurs. Both the nitrate(V) ion NO3- and the sulfate(VI) ion SO42- cannot be oxidized at the anode because both the Group V Nitrogen and Group VI Sulfur are already at their maximum oxidation states of +5 and +6 respectively. It is possible for the oxygens to be oxidized (instead of the nitrogens and the sulfurs), but under aqueous conditions, it is more thermodynamically feasible to oxidize the oxygens from the water solvent, or if you prefer to think of it as, from the hydroxide OH- ions from the auto-dissociation of the water solvent, hence generating O2 gas at the anode, rather than oxidizing the oxygens in the nitrate(V) or sulfate(VI) ions. Therefore for A level purposes, the nitrate(V) and sulfate(VI) ions are to be considered inert and unreactive when carrying out electrolysis of aqueous salts (electrolysis of molten nitrates and sulfates are beyond the scope of the A level syllabus). -
Originally posted by hoay:
What about the following reaction
S2O82- + 2e- 2SO42- +2.01 V
Sulfate ions can be oxidized as the reaction can be reversed. If we increase the [SO42-] ions the E becomes less positive thereby making it feasible to be oxidized.
Secondly, can u through some light on Overpotential.
Oxidation of sulfate(VI) to peroxodisulfate(VI) S2O82- with the 2 peroxo O atoms having an OS of -1, is indeed what I was referring to in my previous post, that is, oxidizing the oxygens rather than the sulfurs. However, as also mentioned in my previous post, the oxidation of the oxygens in the water solvent (and by definition of solvent, it's present in much higher molarity than the sulfate(VI) ions) to generate O2 gas, is more thermodynamically feasible.
For A level purposes, unless otherwise indicated in the question, electrolysis of aqueous sulfates will not oxidize the sulfate(VI) ion into the peroxodisulfate(VI) ion.
Overpotential is a complex matter (as this is a broad term that includes different types of overpotential with competely different and even unrelated underlying chemistry and physics reasons), and is safely beyond the scope of the A level syllabus, and as such I suggest you do not confuse your students with this. On rare occasions when the Cambridge A level exam question includes beyond-the-syllabus concepts such as overpotential, sufficient data (eg. relevant formulae) will certainly be provided in the question to allow the A level student to cope. -
Google honours the chemist Alessandro Volta with a gif depicting the 1st battery (ie. Voltaic cell) ever designed :
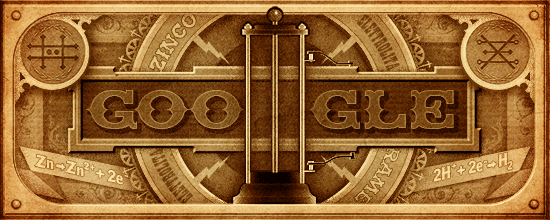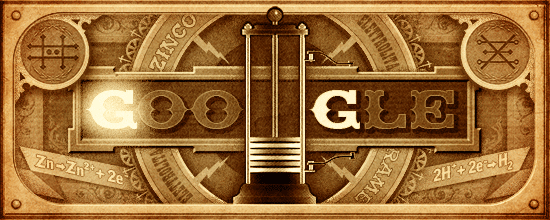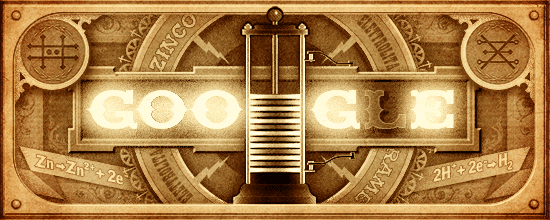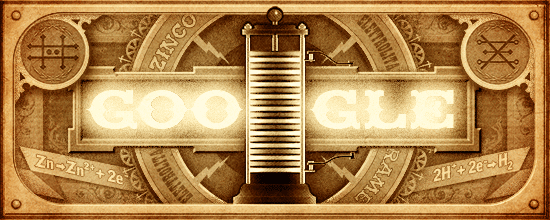
At the anode, Zn is oxidized to Zn2+.
At the cathode, H+ is reduced to H2 gas.
Electrons flow in the external wire from the anode to the cathode, and thus may be harnessed for electrical work.
Interestingly, while for A level Chemistry purposes, a Voltaic cell = a Galvanic cell (ie. an electric cell is an electrochemical cell which generates electricity, as opposed to an electrolytic cell which is an electrochemical cell that uses up electricity), did you know that the chemists Volta and Galvani actually disagreed with each other on the underlying chemistry behind batteries?
http://en.wikipedia.org/wiki/Alessandro_Volta#Volta_and_Galvani
http://en.wikipedia.org/wiki/Luigi_Galvani#Galvani_vs._Volta:_animal_electricity_or_heat_electricity.3F -
Someone asked me on another forum about "Why is the ketone form dominant over the enol form, and what factors could possibly sway the position of equilibrium over to the enol side?"
Bond enthalpies by themselves dictate that the keto form is usually largely favored over the enol form, ie. enol to keto is exothermic, keto to enol is endothermic. Entropy change is negligible.
When the enol form is favored, it's usually contributed to a combination of the following reasons, ordered from the most influential factor to the least influential factor.
1) Conjugation / resonance stabilization energy.
2) Hydrogen bonding :
Intra-molecular hydrogen bonding if a hydrogen bond acceptor group is in close proximity.
Inter-molecular hydrogen bonding if the solvent is aprotic.
3) Poly/multi-substitution of the alkene group in the enol form, due to greater number of stronger C-C sigma bonds with greater % s orbital character. -
Originally posted by Ecxwghnk:
Is there a good alternative to JC notes for H2 Biology?
There are several H2 Biology guidebooks / notes books available at Popular bookstore, written by a couple of different H2 Biology private tutors. They're all pretty good.
NUS Science bookstore has a couple of Uni level Biology textbooks (other than Campbell) that will be useful for the H2 syllabus, particularly Biotechnology textbooks for the applications syllabus. The critically acclaimed Molecular Biology of the Cell - The Problems Book is also available at NUS Science bookstore, and is useful for H3 Biology and H2 Biology students aiming for the distinction A grade.
Free resources @ H2 Biology blog by ex-students for H2 Bio students.
http://jc-biology.blogspot.sg
Free resources @ OwlCove.Sg : lots of summarized notes for all JC H2 subjects, including H2 Biology and H2 Chemistry
http://owlcove.sg/
Lots of internet website links @ H2 Biology private tutor Duncan Ang's webpage
https://sites.google.com/site/jch2biologytuition/9-biology-concepts
-
CityOwl asked : "To draw the experimental set-up to measure boiling point of liquid, should I put the thermometer into the liquid or above it?"
I replied :
The thermometer must be above the liquid. Some sections of the liquid itself (being in almost direct contact with the flame) may often be rapidly heated to a temperature above it's boiling point without properly boiling off, a phenomenon known as superheating, and is thus a less reliable indicator of its boiling point (compared to measuring its vapours). -
Originally posted by hoay:
Distillation and fractional distillation are different in the way that fractional uses compounds having different boiling points while distillation separates impurities from a liquid. Is it correct?
Yes, that's correct. Simple distillation only requires you to separate and obtain 1 volatile species from a mixture containing non-volatile impurity species.
All images below are from Wikipedia : https://en.wikipedia.org/wiki/Distillation
Fractional distillation is required if you wish to separate and obtain 1 volatile species (with the lower boiling point) from a mixture of 2 or more volatile species (with higher boiling points). This process may be repeated (using the same apparatus) to separate and obtain the volatile species with the next higher boiling point.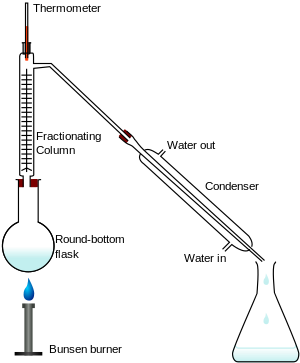
Of course, a more comprehensive, elaborate and extensive setup is used industrially, should one wish to separate and obtain all the different components of a mixture simultaneously, eg. fractional distillation of crude oil : -
^_^
-
Originally posted by hoay:
If we have two alcohols like propanol and butanol then fractional distillation would separate them. Can we use chromatography to separate these alcohols? Where we will use chromatography and not fractional distillation ?
For O and A level purposes, the forms of chromatography used are usually more than analytical than preparative. Meaning that chromatography (for O and A level purposes, mostly paper chromatography and gel electrophoresis, ie. electrochromatographic separation of DNA / RNA / proteins / amino acids) is used for analytical purposes, ie. identifying the components of a mixture, rather than preparative, ie. to separate out large quantities of the individual components.
For O and A level purposes, fractional distillation is hence still the method of choice for preparative separation purposes. However, the limitation of fractional distillation is that it cannot be effectively used to separate non-volatile species (ie. species that are not simple covalent molecules with low boiling points), or volatile species with closely similar boiling points, or azeotropic components (an azeotrope is a mixture of two or more liquid species whose proportions remain unchanged upon distillation), from each other.
Should you be required to separate (for preparative purposes) component species from such problematic mixtures (as described above), then indeed, more advanced forms of preparative chromatography will be more suitable.
Some forms of chromatography are more suited for analytical purposes, while some forms may be adapted for preparative purposes. For a comprehensive list of advanced chromatography techniques (which are beyond the scope of the O and A level Chemistry syllabuses; but of course, Cambridge could always provide relevant information on 1 or more such advanced chromatography technique in an O or A level exam question, and query the candidate on any underlying chemical concepts which are somewhat relevant to the syllabus; as such, candidates gunning for the distinction A grade are advised to explore beyond the basic syllabus requirements for self-enrichment), see
http://en.wikipedia.org/wiki/Chromatography
http://en.wikipedia.org/wiki/Gel_electrophoresis
To answer your question about separating propanol and butanol, yes fractional distillation will suffice. -
As an application question in A level Chemistry, Cambridge has (in previous years) tested the candidate on the analytical separation of amino acids, based on their overall ionic charge (or lack thereof in zwitterionic form) in an agarose gel electrophoresis setup.
A level Chemistry students can visit Jim Clark's webpage and the relevant Wikipedia webpage to prepare themselves for such questions :
http://www.chemguide.co.uk/organicprops/aminoacids/acidbase.html
http://en.wikipedia.org/wiki/Isoelectric_focusing -
Originally posted by hoay:
Two glass vessels M and N are connected by a closed valve.
M contains helium at 20 °C at a pressure of 100000 Pa. N has been evacuated, and has three times the volume of M. In an experiment, the valve is opened and the temperature of the whole apparatus is raised to 100 °C.
What is the final pressure in the system?
BedokFunland JC Solution :
Before valve is opened :
PV=nRT
(100000 Pa) (V1) = (n) (R) (293 K)
This implies nR = 3.413 x 10^2 V1
where V1 = volume of container M
After valve is opened :
PV=nRT
(Y Pa) (V2) = (n) (R) (373 K)
where V2 = combined volume of both containers
Substituting nR = 3.413 x 10^2 V1 into new scenario (since total moles of gas remains the same)
(Y Pa) (V2) = (3.413 x 10^2 V1) (373 K)
(Y Pa) (V2 / 3.413 x 10^2 V1) = (373 K)
Since V2 is (1 + 3 = 4) times the volume of V1,
(Y Pa) (4 / 3.413 x 10^2) = (373 K)
Y Pa = 3.1826 x 10^4 Pa
Originally posted by hoay:Thank you for the comprehensive answer. But is there any other way of doing this and secondly how u know we have to use PV= nRT and other gas relationships as i mentiond.
Boyle's Law, Charles' Law, Gay-Lussac's Law and Avogadro's Law, are all combined into the Ideal Gas Law PV=nRT. Which means (for A level Chemistry, in which we always assume ideal gas behavior for calculation purposes, unless of course when Cambridge occasionally provides in the exam question, info on the more advanced Van der Waals equation, and requires the candidate to apply this in the exam question, as has been asked before in Singapore JC Prelim exams, and makes for an excellent higher order question) you can always just use PV=nRT and ignore the individual sub-laws (and leave other variables alone to cancel out in the later stages of the calculation), like what I did. -
Originally posted by hoay:
Glass is Na2SiO3 any idea why it is transparent?
This question is, of course, totally beyond the scope of any A level Chemistry syllabus in the world.
But if Cambridge were to ask, "Based on your knowledge within your A level Chemistry syllabus, comment on the fact that a particular material, eg. silicate glass, is visually observed to possess optical transparency.".
From the visual observation that a particular material, eg. silicate glass, possesses optical transparency, we can reasonably deduce that the magnitude of the energy difference between the electron orbitals of the atoms or molecules within the material, does not correspond to the visible region of the electromagnetic spectrum. As such, in the event of electron transitions between the orbitals, no light energy corresponding to the visible region of the electromagnetic spectrum is absorbed.
That's as far as the A level Chemistry syllabus is concerned (if this was a A level Physics question, no doubt there would be something relevant in the A level Physics syllabus for the student to make reasonable comments as well). Anything beyond this would be beyond the scope of the A level Chemistry syllabus, and thus beyond the scope of my personal and professional interest as well. If you're looking for more info on this, you can ask this question on a University Physics + Chemistry inter-disciplinary forum. -
'A' Level H2 Chemistry Qn
1.15 g of a metallic element reacts with 300 cm3 of oxygen at 298 K and 1 atm pressure, to form an oxide which contains dinegative O2– anions. What could be the identity of the metal?
MCQ options : A calcium - B magnesium - C potassium - D sodium.
My BedokFunland JC Solution :
Let the molar mass of the metal be y grams
No of moles of metal present = 1.15 / y
Using PV=nRT, molar volume of gas = 24.45 dm3.
Hence moles of gas present = 12.27 x 10^-2 mol.
By stoichiometry, moles of dinegative O2- anions generated = 24.54 x 10^-2 mol
Assuming the metal cation has a unipositive charge :
moles of metal cation = 2 x moles of oxide anion
Hence 1.15 / y = 49.08 x 10^-2, and y = 23.43 g
Assuming the metal cation has a dipositive charge :
moles of metal cation = moles of oxide anion
Hence 1.15 / y = 24.54 x 10^-2, and y = 46.86 g.
Conclusion : Comparing against the molar masses and ionic charges of the 4 metals listed in the options, the closest match and hence the best answer, is Na (sodium).
Commentary : if we had used 24dm3 as the molar volume of gas, as might have been intended by the question author, we would obtain molar masses of 23.0g and 46.0g for unipositive and dipositive ionic charges respectively, making Na (sodium) a more obvious answer. However, 24dm3 is actually the molar volume of a gas to 3 sig fig when room temperature is taken to refer to 20 deg Celsius, rather than 25 deg Celsius (both may be described as "room temperature", itself an ambiguous term). Alternatively, recalcitrant folks might argue that 24dm3 is still correct for 25 deg Celsius, if rounded off to 2 sig figs. Yeah well, 2 sig fig is just bad math, particularly when the question itself gave the sample mass of the metal to 3 sig figs. This is commonly misunderstood (even by school teachers), but will usually not be much of a problem in the actual A levels, as Cambridge will usually specify along the lines of"You may assume the molar volume of a gas under these conditions to be 24dm3" *or* explicitly require you to use PV=nRT. -
Gohby asked :
Hi,
I have some H2 Chem MCQ questions that to discuss/solicit for opinions. Any help would be much appreciated, cheers!
Q1: Modified CJC Prelims 12:
The Thermit Reaction involves mixing iron(III) oxide with aluminium powder in a crucible, with a suitable fuse to start the reaction. The reaction is as follows:
Fe2O3(s) + 2Al(s) → Al2O3(s) + 2Fe(l)
The fuse is first ignited, where it will burn in oxygen, forming the oxide with a large release of heat required for the Thermit reaction to take place. The commonly used material for the fuse is a clean magnesium strip. Which of the following helps to explain why a strip of magnesium is suitable to be used as a fuse?
1: The numerical value of the enthalpy change of formation of magnesium oxide is very large.
2: The strip increases the surface area for magnesium to react with the oxygen at a faster rate.
Ans: 1 and 2 are correct
Remarks: I think that 1 and 2 are not precise enough to serve as explanations.
For 1, the numerical value of the enthalpy change of formation of magnesium oxide being very large does not explain its suitability, since it does not tell us anything about the sign of the enthalpy change. The enthalpy change of formation should have been large AND negative to generate enough heat to kick start the Thermit Reaction.
For 2, where is the basis of comparison to say that the strip increases the surface area for magnesium? I could argue that (compared to magnesium power), the strip is not as suitable to be used as a fuse because the surface area is lower.
Q2: AJC Prelim 2012
The Gibbs free energy change of a system determines whether a reaction is spontaneous, while the equilibrium constant indicates the extent of reaction. What does the following pair of values for a reaction system indicate?
Values:
ΔG –50.8
Kc 5.80x10^8
A: No reaction
B: Position of equilibrium lies to the left
C: Some extent of reaction
D: Reaction goes to completion
Ans: D
Remarks: I note that the reaction is spontaneous and the Kc is a very large value, but shouldn’t the answer be C, given that the reaction is still after all, a reversible reaction (and therefore it does not go to completion)?
Q3: How do I know that the following equation: H2O (l) → H+ (aq) + OH- (aq) has a positive enthalpy change of reaction?
---------------------------------------------------------------
UltimaOnline replied :
For Q1, you're right of course. At the same time, it's not feasible to have detailed, mini-essays as options for an MCQ. Hence, your explicit points are to be taken as implied by the summarized options. The student is to select the best possible options in any case.
Edited to add : while you're right to say the option 1 could be improved to explicitly specify the formation enthalpy of MgO needs to be exothermic, nonetheless (or it could also be the question's intention to test that) the candidate should be able to draw the inference from MgO's extremely strong ionic bonds (due to high cationic and anionic charge densities) that the formation enthalpy would naturally be strongly exothermic and thus thermodynamically favourable. While Singapore JC Prelim paper questions are notoriously ambiguous and debatable, but Cambridge isn't always much better (every year there's always at least a couple of debatable questions in which JC teachers disagree on what's the best answer, and different TYS authors provide different answers), so advise your (more able) students to always psychologically profile the Cambridge question setter's intention, and choose the best available options.
PS. Check out this YouTube video of a Chemistry teacher demonstrating the Thermite reaction : https://www.youtube.com/watch?v=qqvQwfH_wGQ
For Q2, notice that the Kc value is so large, that the reaction is mathematically 99.999% complete, which when rounded off to 3 sig fig, is hence to be regarded as 100% complete. As always, choose the best available option(s).
For Q3, the forward reaction involves only covalent bond breaking, and is thus endothermic. Of course, ion-dipole bonds are formed exothermically, but are of a significantly lesser magnitude compared to covalent bonds. Even if the question were to involve the hydroxonium ion (instead of simply H+), the final answer in this context would still be the same. -
Originally posted by gohby:
Thank you very much, UltimaOnline. :)
I have some questions on Ideal Gases:Q1: What is the pressure (in Pa) of a sample of hydrogen gas that has density of 8gm-3 at 300 degrees celsius?
Answer: (573x8x11.2x101)/273
Remarks: Pressure = (DensityxRxT)/Mr = (8x8.31x573)/4?? Why doesn't this work?
Q2: Which changes in conditions would not increase the voulme of a fixed mass of gas?
Pressure/Kpa Temperature/K
1. Halved Halved
2 Doubled Halved
3 Halved Doubled
Answer: 1 & 2
Remarks: According to the equation pV=nRT wouldn’t the answer be 1 only?
Q3: 1dm³ of gas X weighs 1g and 1dm³ of gas Y weighs 5g under the same conditions of temperature and pressure. Which of the following statements are correct?
-
The ratio of the Mr of X to Y is 1:5
-
The average velocity of the molecules in gas x and gas Y are the same at the same temperature
-
The number of molecules in Y in 1dm³ is 5 times the number of molecules of X in 1dm³
Answer: 1 only
Remarks: Isn’t the answer 1 and 2? Shouldn't the average velocity of gaseous molecules be the same at the same temperature? 3 is wrong because it should have been 1.5 times instead of 5 times.
Totally welcome, Ghoby :)
Q1: What is the pressure (in Pa) of a sample of hydrogen gas that has density of 8gm-3 at 300 degrees celsius?
I would advise students to work on density separately from the ideal gas formula.
Density = Mass / Volume, which implies Mass = 8 x 1 = 8g in 1 m3, which implies 4 mol of H2 gas in 1 m3.
PV=nRT, which implies P=(nRT)/V, which implies P = ( 4 x 8.314 x 573 ) / 1 = 1.9056 x 10^4 Pa.
Of course, if it's an MCQ qn, and the 4 options only show working instead of the final answer (doncha just hate these type of silly MCQs), then you've no choice but to do ur best to backward engineer and make an educated guess on (what's on) the mind of the question setter, how *he* does *his* own working to arrive at the answer.
Looking at the given answer you posted, he's using molar volume of ideal gas at stp = 22.4 dm3.
Q2: Which changes in conditions would not increase the voulme of a fixed mass of gas?
Pressure/Kpa Temperature/K
1. Halved Halved
2 Doubled Halved
3 Halved Doubled
It's a trick qn. Option 1 has no change in the volume. Option 2 decreases the volume. Either way, the volume doesn't *increase*.
Q3: 1dm³ of gas X weighs 1g and 1dm³ of gas Y weighs 5g under the same conditions of temperature and pressure. Which of the following statements are correct?
The ratio of the Mr of X to Y is 1:5
The average velocity of the molecules in gas x and gas Y are the same at the same temperature
The number of molecules in Y in 1dm³ is 5 times the number of molecules of X in 1dm³
This MCQ tests your fundamentals of Physics. Kinetic energy = 0.5 x mass x velocity^2
At the same temperature, all ideal gases have the same average kinetic energy. But because different gases have different masses, hence the average velocities of different gases are different, even as the average kinetic energy of different gases are the same, at the same temperature.
Statement 3 is incorrect, because assuming ideal gas behavior, the same volume of both gases (at the same temperature and pressure) would contain the same no. of molecules (whether mono-atomic or poly-atomic, is irrelevant in this context). -
-
Originally posted by gohby:
Thank you very much UltimaOnline.
I have further questions on Chem Bonding/Atomic Structure.
Q1: Beryllium difluoride reacts readily with trimethylamine, (CH3)3 N to form a stable addition product. Nitrogen trifluoride has no reaction with trimethylamine.
Which of the following statements are true?
-
The molar ratio for the reaction between beryllium difluoride and (CH3)3 N is 1:1.
Remarks: The answer shows that 1 is false, but why? Isn’t the addition product formed with a dative bond between N and Be, thereby giving N a stable octet.
Q2: What type(s) of bonding occur in NaOCl?
-
Van der Waals forces
Remarks: The answer shows 1 is wrong, but isn’t there Van der Waals forces in OCl-?
Q3: Which of the following statements is wrong?
A: The melting points of the Group I hydroxides increase with increasing relative molecular mass.
I know A is wrong because the melting point decreases, but I am pondering why that's the case. Is it because the ionic bond between Gp I cations and the hydroxide ions become weaker as they go down the group, given that the effective nuclear charge of the valence electrons of the bigger Gp I metals like Cs is lower, thereby increasing the covalency of the ionic bond and thereby weakening the strength of the ionic bond?
Q4: At one stage in the radioactive decay of the osope 235 92 U, the isotope 211 82 Pb is present. How many alpha particules and beta particules will be emitted in the decay? An alpha particle is 4 He²+ and a beta particle is 0 e-
2 -1
A: 2 alpha particles and 6 beta particles
B: 6 alpha particles and 2 beta particles
C: 6 alpha particles and 6 beta particles
D: 12 alpha particles and 12 beta particles
Remarks: Why is there -1 in the configuation of the electron 0 e-, as if an electron contains one missing proton? -1
I would have thought that there would be 5 alpha particles being emitted since the difference in protons between U and Pb is 10.
No problem :)
Q4. From 235,92,U to 211,82,Pb we note that 10 protons and 14 neutrons are emitted, which has a total mass of 24, and an overall charge of 10+. The only option which fits this, is option B. About your remark : because for this question, the question specifies that beta particles are electrons (instead of positrons), and as such an electron beta particle is a particle that contains zero protons and negligible mass.
Q3. Ionic bonds weaken because cationic charge densities decrease down the Group. Degree of covalency further decreases, not increases (since the magnitude of electronegativity difference between the metal and oxygen increases). Finally, effective nuclear charge does *not* decrease down the Group, as is often wrongly taught by Singapore JC teachers. The real reason for decreasing electronegativity and decreasing endothermic ionization enthalpy down the group is due to Coulomb's law (ie. increasing atomic radius).
Q2. Van der Waals forces exist between molecules. Because sodium hypochlorite (Latin name) or sodium chlorate(I) (Stock name) is an ionic compound, the electrostatic attractions between the ions are named ionic bonds, rather than van der Waals forces. To address your question directly : even if it were a neutral molecule rather than an ion, van der Waals forces exist intermolecularly, ie. between molecules, not intramolecularly, ie. within molecules.
Q1. Because trimethylamine is a monodendate Lewis base (ie. only 1 dative bond can be donated per N atom, and only 1 N atom is present per trimethylamine molecule ; note it is incorrect to say "because the N atom has only 1 lone pair" as often wrongly taught by Singapore JC teachers, because even if 2 lone pairs were present, eg. H2O ligand, each O atom can only donate 1 dative bond because to donate both lone pairs as dative bonds would incur an unacceptably destabilizing dipositive formal charge), and because the electrophilic Be atom of the beryllium difluoride Lewis acid has only 2 existing bond pairs, therefore stoichiometrically, 2 moles of trimethylamine Lewis bases are required to nucleophilically add (ie. donate dative bonds) unto each mole of beryllium difluoride Lewis acid molecule, in order for Be to have 4 bond pairs and hence a stable octet noble gas configuration. -
-
Originally posted by hoay:
the heat change measured at constant pressure is Enthalpy change. It tells the energy of the system before and after the reaction. Then why we need entropy? What does it tells?
Entropy change tells you about the measure of disorderliness before and after a chemical reaction. Why do you need entropy, in addition to enthalpy?
When considering the choice of a job, profession or career, you need to consider both job satisfaction, as well as how well it pays you. Both factors are important.
Of course, which factor is more important at this point in time to you, also depends on your family financial needs, eg. how much you need to support your family.
Your family financial needs is represented by temperature. Job satisfaction is represented by enthalpy. Job pay is represented by entropy.
If your family financial needs (temperature) is high, then job pay (entropy) takes priority over job satisfaction (enthalpy). If your family financial needs (temperature) is low, then job satisfaction (enthalpy) takes priority over job pay (entropy).
For the choice of a job / career / profession that successfully suits you and allows you to proceed forward in your life, both job satisfaction and job pay need to be considered, and is affected by a 3rd variable, family financial needs. For a chemical reaction to successfully proceed in the forward direction (ie. for Gibbs free energy to be negative and thermodynamically feasible and favorable), both enthalpy and entropy need to be considered, and is affected by a 3rd variable, temperature.