BedokFunland JC's A Level H2 Chemistry Qns (Part 2)
-
You're right that the position of equilibrium has shifted to the RHS, generating a *slightly* greater number of moles of RHS species at equilibrium.
However, because the volume has doubled (ie. *greatly* increased), hence even with the *slight* increase in moles of RHS species, the new equilibrium molarities of the RHS species still decrease (though the decrease is not as much as the LHS species, whose moles and molarities *both* decrease).
Originally posted by Light5:Q.) http://gceguide.com/papers/A%20Levels/Chemistry%20(9701)/9701_s15_qp_11.pdf
In q 6 of the above paper, if volume is increasing from 1 to 2 , then pressure should decrease therefore equilibrium must shift to the RHS to increase No of Moles of PCl3 and Cl2 and decrease No of Moles of PCl5. This is reflected in both answer choices A and B yet the examiner has given B as the answer in the marking scheme? Can you please clarify this?
-
Originally posted by Mrworry :
A compound C3H6O2 which produces CHI3 and reduce Fehling solution. Does CH2(OH)COCH3 fulfill this requirement?
UltimaOnline aka BedokFunland JC replied :
Yes it does. This qn would kill A level H2 Chem students though ;Þ
Originally posted by Mrworry :
c3h6o2 which does not produce triodomethane but reduce fehling soln
CH2(OH)CH2OCH
Does this fulfill the req, if not why?
UltimaOnline aka BedokFunland JC replied :
Your structure is in error. Ensure that you satisfy the valencies of O and C such that all atoms have a stable octet but no formal charge.
Update : MrWorry, you probably got the correct structure, but you expressed the condensed structural formula wrongly. You should write it as follows (ie. the way I wrote it below).
The correct answer should be : CH2(OH)CH2CHO
Originally posted by Mrworry:Xiexie~
Btw I am not sure why but the answer given for first part is CH3COCOH and second part is CH3OCH2CHO. @.@
Are my answers accepted?
UltimaOnline aka BedokFunland JC replied :
Yes, your answers are fine. The given answer is fine for the 2nd qn, but wrong for the 1st qn (count the no. of H atoms). -
bestrobber97 asked :
Hello Ultima,
I'm taking my A's this year and need some help with a question. In Paper 3, Q3(d), why can't P be CH3CH(Cl)CH(OH)CH2COCH3 ? And on a side note, what are the 3 isomeric products that dehydration of P would give? I know the 2 obvious ones, but can't seem to think of the third :(
Thanks in advance :)
<2014 RJC P3 Q3
Compound P, C6H11O2Cl, reacts with 2,4-dinitrophenylhydrazine to form an orange precipitate and decolourises hot, acidified potassium manganate(VII). When compound P is heated with excess aqueous sodium hydroxide, compound Q, C6H12O3, is formed. Aqueous iodine is subsequently added to the hot mixture of this reaction and compound R, C4H4O5Na2, is formed. P reacts with excess concentrated sulfuric acid at 170 °C to give three possible isomeric products S, T and U. S, but not T and U, reacts with excess ammonia to form an amine. Suggest the structures of P, Q and R, explaining your reasoning.
P cannot be CH3CH(Cl)CH(OH)CH2COCH3 because the qn stated that from Q to R (when reacted with alkaline aqueous iodine), 2 O atoms are added, and 2 C atoms are removed, meaning that 2 groups separately underwent the iodoform reaction. Based on degree of unsaturation (1 for Q and 2 for R), you can deduce that 1 group must have been CH3CH(OH)R and the other group must have been CH3COR.
Hence, P must have been CH3CH(OH)CH(Cl)CH2COCH3.
S is CH2=CHCH(NH2)CH2COCH3, being a terminal alkene has no geometrical isomerism.
T and U are cis & trans (or E & Z) CH3CH=CClCH2COCH3, with the vinyl halide C-Cl bond of the resonance hybrid having partial double bond character, which is thus strong and does not cleave readily, and hence T & U are resistant to nucleophilic substitution by NH3. -
Originally posted by Light5:
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_w14_qp_43.pdf
^Q3(d) .... I know that the observed colour is the complement of the colour absorbed but i cant seem to understand how to interpret this graph....there is high absorption in the blue-violet region which should yield a green-yellow colour according to the colour wheel. Furthermore, the high absorption in the orange-red region should yield a blue-green colour. I cant understand what the final colour will be since there are so many being absorbed and so many combinations possible.
MS : http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_w14_ms_43.pdf
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_w09_qp_41.pdf
^Q3(b) from above paper : Same issue as before...a range of wavelengths is being absorbed. For the Copper-Ammonia Complex, max absorption is in green-orange region which yields violet,dark blue and blue according to colour wheel. For the aqua-copper complex there is no absorption at all in both blue AND green regions so shouldnt the colour observed be bluish-green. The MS states it will be pale blue but how can i interpret it by only using the graph.
MS: http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_w09_ms_41.pdf
Please give a detailed explanation.
Thank You Very Much !
1st paper : Cambridge is challenging the candidate to think more directly : since almost all colors of the spectrum are absorbed except green / yellow, the color observed is green / yellow. You don't even need to use the color wheel to check the complementary color.
2nd paper : Cambridge is testing whether the candidate notices the exact phrasing of the question : (i) says "State" (ie. from memory, without using spectra graph), while only (ii) then says "Using the spectra above". -
Originally posted by BCME:
Hi, the following qns are asked with reference to VJC_P3_2014
1. 1(a)(iii) not very sure how to calculate the ratio
2. 2(d) how do we know how to construct the equation between P and NaOH?
3. 3(i) why KMnO4 cannot be used?
4. 4(b) I know that J contains COOH. However when it is reduced won't it be converted to primary alcohol and alcohol will react with conc sulfuric acid so I am a bit confused.
Thanks
Q1. At equilibrium, by definition, no electric current flows through the circuit, Gibbs free energy change = Cell Potential = 0 volts.
Kc = [Products] / [Reactants]
Hence Kc = [Mg2+] / [Cu2+]
Since Mg2+ is the RHS product and Cu2+ is the LHS reactant.
Q2. This is a disproportionation reaction in which highly reactive and unstable P4, undergoes (base-promoted) hydrolysis, and is simultaneously oxidized to oxo-acidic hypophosphorous acid (immediately deprotonated into its conjugate base salt), and reduced to hydridic-basic phosphine.
Note the electronegativities of O > P > H, which will help you to better understand the oxidation-to-acidic and reduction-to-basic nature of the hydrolysis reaction.
The chemistry and mechanism for this reaction is, of course, beyond the A level syllabus. But sufficient clues are given in the question, specifically data that enables you to calculate the empirical formula of species C, and of course, the textual description of the reaction of A + NaOH + H2O* --> B + C.
* Whenever there is (aq), eg. NaOH(aq), the astute or distinction student will be open to the possibility of hydrolysis (ie. reaction with H2O), especially in acidic or alkaline conditions. Eg. In Organic Chemistry, whenever KMnO4 / H+ or KMnO4 / OH- is used, don't just think oxidation, but open yourself to the possibility of acidic or alkaline hydrolysis of any ester / amide / nitrile / etc groups simultaneously occurring as well, in challenging deductive elucidation questions.
Q3. Heating under reflux with acidified KMnO4, the alkyl side chains of the benzene rings (there are 2) would be oxidized, yielding ortho-phthalic acid (from benzene ring on the left), benzoic acid (from benzene ring on the right), 2-methylpropandioic acid (centre fragment), and (from the methyl group beta to the benzene ring on the left) carbonic(IV) acid (which decomposes into CO2(g) and H2O(l) with thermodynamically favourable positive entropy change, and as predicted by Le Chatelier's principle).
Q4. While J is a primary alcohol, its only beta C atom happens to be a tertiary C atom, ie. no beta H atom (required for elimination together with the alpha OH group during dehydration of alcohols to alkenes) is present. This clues you in on the branched structure of fragment J, and hence of parent G as well. -
Originally posted by Mrworry:
H202 + 2I- + 2H+ →2H20 + I2
Initial [I-] = 0.001 mol/dm3
[H202] = 0.05 mol/dm3
Half life = 90s
Rate = k [H202] [I-]
Predict half life iodide in expt 2 where [H202]= 0.10 mol/dm3 and [I-] = 0.001.
Why the half life is deduced to 45 seconds?
Isnt halflife constant to concentration of reactant if it is 1st order to the said reactant?
Half-life of 1st order reactant iodide ions, is halved because molarity of other reactant, 1st order reactant H2O2, is doubled in the new experiment.
Half-life for 1st order reactant is indeed constant regardless of how its own molarity changes, but *ONLY IF* the molarities of all other non-zero order reactants remain unchanged (here molarity of other reactant H2O2 changes), *OR* if it is overall 1st order, *OR* if it is overall pseudo 1st order (ie. having any other non-zero order reactants present in large excess so any changes in the molarities of these other reactants as the reaction proceeds are negligible).
If you drastically change the molarity of another non-zero order reactant going from experiment 1 to experiment 2, such as by doubling it, even if already present in large excess in experiment 1, the rate of reaction will still increase (eg. doubled if 1st order, or quadrupled if 2nd order, etc) and hence the half-life of the original 1st order reactant will still be halved (or quartered, etc) such as in this question you've typed out. -
Originally posted by gohby:
Hi UltimaOnline,
I have some “application� questions this time.
Q1: http://img.photobucket.com/albums/v700/gohby/Chemistry/Hofmann_zpsbhaiqwk4.jpg
Remarks: The answer is A. Firstly, am I right to say that a quaternary, rather than a tertiary amine intermediate would be formed instead? Secondly, without understanding the Hofmann elimination mechanism, how would I be able to arrive at option A? If I were to try to extrapolate from the example given, A wouldn’t have been the answer since the example shows that nitrogen has been eliminated.
Q2: http://img.photobucket.com/albums/v700/gohby/Chemistry/ether_zpsld41to9s.jpg
Remarks: The answer is C. Is this a reasonable question to ask in the syllabus (since ethers are not in the syllabus)? I do not understand why there would be nucleophilic substitution taking place for the CH2Br but elimination occurring instead for CH2CH2Cl.
Q3: http://img.photobucket.com/albums/v700/gohby/Chemistry/Claisen%20condensation_zpsqydyie04.jpg
Remarks: The answer is A. Why? Is there a way to arrive at the answer without understanding (the sophisticated) mechanism for Claisen condensation?
Q4:
http://img.photobucket.com/albums/v700/gohby/Chemistry/Least%20likely_zpsdxb98tfr.jpg
Remarks: The answer is C. A is likely to occur since it’s part of the nucleophilic addition reactions for carbonyl compounds. B is likely to occur since it’s FRS. I presume C is unlikely to occur because the positive charge is stabilised by resonance. However, I would have chosen D - D is a primary halogenoalkane, so SN2 nucleophilic substitution reactions are more likely, but D shows the first step of the SN1 reaction. Though I understand that the benzene ring is a bulky substituent that would favour the SN2 reaction happening instead, how would I be able to compare the likelihood of D or C occurring?
Lastly, wrt your response on QI in the previous post,
QI. Yes if your suggested answer is one of the options, go for it. But if not (since the exact type of oxidation isn't specified and is obviously not in H2 syllabus), just choose the best, most feasible, answer.
I would have thought that A was the best and most feasible answer since 1,2,4,5-benzenetetracarboxylic acid is one of the choices. I would have at least entertained B as a feasible option if not for the presence of the ketone. I did not find it feasible that the alkane can inexplicably be oxidised to a ketone..
Thank you very much, really appreciate your help! :)
Such higher order-type qns are designed to be do-able only by distinction students, and as such for these qns, the boundaries between in-syllabus and beyond-syllabus is blurred.
Many of these qns will require the ability to draw mechanisms based on understanding (not blind memorization), which rules out the majority of Singapore JC H2 Chem students.
Typically, only students who actually understand and can draw mechanisms for reactions not previous encountered (ie. H3 Chem, Olympiad Chem, and BedokFunland JC students), can correctly answer these questions with a correct understanding of the underlying mechanisms.
True, some (not all) of such qns can still be guesstimated using pattern-recognition, but that would be more of an IQ test (or for most JC students, a random game of roulette) rather than actually understanding the underlying chemistry involved.
Q1. With the mechanism drawn, the answer becomes obvious. SN2, deprotonation, SN2, E2. Go ahead and draw out the mechanism to explain to your students.
Q2. Dehydrohalogenation to generate an alkene group cannot occur for the methyl side-chain as the adjacent (benzene ring) C atom does *not* have a H atom present to be eliminated.
Q3. This qn requires understanding and drawing out of the mechanism (you should go ahead and draw out the full mechanisms to explain to your students, but advise them to skip such qns in the actual A levels Paper 1 first, and go back to them only after they've completed the rest of the paper, in the interest of exam-smart time-management). Since the qn specified a mixed / crossed (instead of self) Claisen condensation, it could either be ethyl ethanoate as nucleophile attacking ethyl pentanoate as electrophile, or ethyl pentanoate as nucleophile attacking ethyl ethanoate as electrophile. Either way, all 4 options given in the qn are wrong. Draw out the mechanism and you'll see the structures of the 2 possible products.
Q4. Option C shows the tetrahedral sp3 hybridized intermediate of a benzene ring during electrophilic aromatic substitution. The Br- must compulsorily act as a Bronsted-Lowry base to carry out deprotonation, to restore the benzene ring's aromaticity (temporarily lost during electrophilic aromatic substitution), to re-acquire the resonance stabilization energy as dictated by thermodynamics and Gibbs free energy. This is in the H2 syllabus under "Candidates must be able to explain why the benzene ring undergoes electrophilic aromatic substitution instead of electrophilic addition".
QI. 1st of all, your suggested 1,2,4,5-benzenetetracarboxylic acid is NOT a product under option A. 2ndly, 1,2,4,5-benzenetetracarboxylic acid wouldn't be a product in any case, as oxidation of akyl side chain of benzene ring requires a benzylic H atom, which isn't present here. As for "alkane inexplicably oxidised to a ketone", it's not so inexplicable when you consider the side-chain oxidation of benzene ring by KMnO4 (which *is* within the H2 syllabus), extrapolated out in this qn's molecule. So it's not "alkane inexplicably oxidised to a ketone" per se, but rather "alkyl side chain of benzene explicably oxidised to a ketone".
No problem, Gohby, you're totally welcome. Keep your questions coming (thousands of silent forum lurkers, quite certainly both students as well as teachers/tutors, also follow and benefit from our discussions here). -
Originally posted by BCME:
Hi, with reference to YJC_P3_2014
2 (b)(iii) Why will NaBr and conc H2SO4 react with the alkene group?
Because alkenes, being electron-rich Lewis bases, are both nucleophilic and Bronsted-Lowry basic.
Mechanism : alkene double bond attacks H+, then Br- attacks C+. -
Originally posted by gohby:
Thank you for your help UltimaOnline :)
I have some more questions here:
Q5:
http://img.photobucket.com/albums/v700/gohby/Chemistry/crackin_zps1k1zqfbz.jpg
Remarks: How do I establish which equation is wrong?
Q6:
http://img.photobucket.com/albums/v700/gohby/Chemistry/imine_zpsgxwtogli.jpg
Remarks: The answer is 1 only. However for option 3, when I add NaOH to the mixture, according to the equation NH3+H2O ⇄ NH4+ + OH-, wouldn’t the concentration of ammonia increase, thereby increasing the yield of imine?
Q7:
http://img.photobucket.com/albums/v700/gohby/Chemistry/deuterium_zpsytr2ghdh.jpg
Remarks: The answer is 8. I could only find the 8 isomers if the question states that it was the molecular formula, instead of the structural formula that was shown (because if the propyl substituent group could be branched there would be more isomers). However since the question states that it is a part-structural formula, I would have to assume that the propyl substituent group is unbranched, am I right?
Q8:
http://img.photobucket.com/albums/v700/gohby/Chemistry/cream_zpsvuoebonr.jpg
Remarks: How do I ascertain what is Y and Z from the table?
No prob, Gohby :)
Q5. The qn is in error. What the AJC teacher meant was "in the absence of a catalyst" (ie. thermal cracking, not catalytic cracking). In which case, option C is the answer, as based on the mechanism (ionic vs free radical), branching in products are a feature of catalytic cracking (due to carbocation rearrangement via hydride and methanide shifts). To achieve the branched product in option C via thermal cracking involves the participation too many highly unstable methyl radicals (unlikely to be generated based on the long chain reactant), and/or highly unfeasible free radical rearrangements, both requiring overly high activation energies, and are thus both thermodynamically and kinetically unfeasible. Finally (tell your students this only after going through everything above and enjoying their worried looks), cracking isn't in the H2 syllabus, so Cambridge won't ask such qns in the 1st place.
Q6. Another problematic qn. There are 2 reasons that outweigh your (reasonable enough) assumption. 1stly, with the addition of NaOH, the overall yield of the imine decreases due as some of the aldehyde will be used up to undergo Aldol side reactions, hence shifting the position of equilibrium of the original reaction to the LHS. 2ndly, the mechanism to generate the imine requires a fairly neutral pH (adding NaOH makes it too alkaline for the proton transfers that need to occur during the mechanism for generation of the imine). Yes, you would say this is unfair to H2 students, which is why Cambridge won't set such a qn in the first place.
Looking at problematic Q5 and Q6, remember what I've always said about not trusting Singapore JC Prelim papers too much?
Q7. C3H7 side chains could refer to either propyl or iso-propyl.
Q8. The key phrase is "after some time". If you wait long enough, both the alkyl dibromide and the bromo acyl bromide will yield the same ppt. But if you only wait a short while (ie. after some time, not too long), the bromo acyl bromide will yield a greater mass of ppt, as acyl halides undergo hydrolysis instantaneously at rtp, while akyl halides require a much higher Ea, a much higher temperature, a much longer time. -
Originally posted by gohby:
Hi UltimaOnline,
Thank you for your help. :) I have another set of questions to ask -
Q9.
It has been noted that prolonged exposure of wine to the atmosphere will result in the wine becoming “vinegary�. Therefore, wineries attempt to prevent such exposure by storing the wine in a mixture of carbon dioxide and nitrogen. How does this mixture prevent the “vinegary� taste from occurring?
1 Oxidation of ethanol into ethanoic acid will be prevented.
2 The acidity of the wine will be decreased.
3 The amount of carbon dioxide dissolved in the wine will be increased.
Answer: 1 only
Remarks: I can infer that the vinegary taste arose as a result from oxidation, but how do I conclusively strike out the other options?
Q10.
H2NCH2CH2CH2CO2H
Which statements about the above compound are correct?
1 It is a 2-aminocarboxylic acid.
2 It is soluble in water due to zwitterion formation.
3 It migrates to the anode of an electrolytic cell at pH 12.
Answer: 1 and 2.
Remarks: I think the answer should be 2 and 3 instead. Isn’t the compound a 3-aminocarboxylic acid? Additionally, at pH 12, the carboxylic acid will react with the base to form a carboxylate ion, which should migrate to the positive terminal (anode) of the electrolytic cell.
Q11.
Which reactions yield a carbon compound incorporating deuterium, D?
1 CH3COCH3 --(NaOD, I2, D2O)---->
2 CH3CO2CH3 --(NaOD, D2O) ----->
3 CH3CH2CN --(NaOD, D2O) ------->
Answer: 1 and 2 only.
Remarks: Why would the product in reaction 1 contain deuterium? Are the products of reaction 1 CH3COO- and CHI3?
Q12.
SAJC/PRELIM2009/P1/Q23
Which of the following pairs of compounds can be attacked by cyanide ions via
nucleophilic reactions?
A CH3CH2Cl and CH3COCH2CH3
B CH3CH2Cl and CH3COOCH3
C C6H5Cl and CH3COCH2CH3
D C6H5Cl and CH3COOCH3
Answer: B
Remarks: I know that C and D are wrong because of chlorobenzene, but I would have assumed that the ketone in A can be attacked by cyanide ions nucleophilically as per nucleophilic additions of carbonyl compounds. However, am I also right to say that the C in the ester group is also susceptible to nucleophilic attacks as it is rather δ+?
Q9. Option 2 is wrong because CO2 being an covalent, electrophilic, acidic oxide, will actually (directly) increase, not decrease the acidity (of course indirectly, when the acidity is increased as a result, the bacterial population is consequently reduced, thereby making the pH overall still less acidic compared to if the bacteria thrived and oxidized the ethanol to ethanoic acid. But that's beyond H2 Chem syllabus, and that'll just be a sadistic "heads-I-win-tails-you-lose" MCQ setup by the examiner). It is the bacterial oxidation of ethanol to ethanoic acid (in the presence of atmospheric oxygen), which is really what gives the sour vinegary taste, rather than dissolving CO2 to generate carbonic(IV) acid (which is what makes soft drinks fizzy, eg. Coca Cola). Option 3 is wrong because it's true but irrelevant. As mentioned earlier, hydrolysis of CO2 will generate carbonic(IV) acid, which increases the acidity but does not *directly* (sadistic examiners notwithstanding ; indirectly it's arguable that it does) help to prevent the oxidation of ethanol to ethanoic acid in presence of atmospheric oxygen (and it is the ethanoic acid that gives the vinegary taste). The primary purpose of forcing CO2 and N2 in is to eliminate O2. Of course, if it were as simple as that, then it'll be better just to use N2. The CO2 present is additionally meant to reduce the pH to levels more acidic than that required for bacteria (which oxidize ethanol to ethanoic acid) to thrive. And it sure doesn't hurt that the dissolved CO2 or carbonic(IV) acid adds a nice fizzy touch to the alcoholic beverage.
Q10. You're correct. Don't trust Singapore JCs.
Q11. In the reaction mechanism for the iodoform reaction, the 1st 3 hydroxide ions function as Bronsted-Lowry bases, while the 4th hydroxide ion functions as a nucleophile, generating RCOOD and CI3-. The CI3- then functions as a Bronsted-Lowry base to abstract a D+ ion from either RCOOD or the solvent D2O. Either is possible, and since the qn ensured both the hydroxide ions and solvent water consist of D instead of H, it's not an issue here.
Q12. Potentially, both ketones and esters may react with cyanide ions (being a strong nucleophile). However, as far as the H2 syllabus is concerned, only ketones (and not esters) react with cyanide ions. The rationale for this is that ketones (and aldehydes) are significantly more electrophilic compared to esters (or for that matter, carboxylic acids and amides as well). The underlying rationale for this rationale, which is not taught at either H2 or even H3 (and not well understood even by most JC teachers), is that the partial positive charge at the electrophilic carbonyl C atom in ketones is due to both induction and resonance (ie. the resonance hybrid has an electron-deficient carbonyl C atom as a consequence of the carbonyl O atom withdrawing electrons by resonance), while the partial positive charge at the electrophilic acyl C atom in esters is due only to induction (ie. the resonance hybrid does not have an electron-deficient acyl C atom as a consequence of the acyl O atom withdrawing electrons by resonance, due to the adjacent O atom that simultaneously donates electrons by resonance to the acyl C atom).
If at this point you wanna retort, "but the Singapore JC mark scheme said it is the ester that kena attacked leh, not the ketone!", then see my Q10 above.
Qn about Iso-Propyl. If it were full-structural formula : when C3H7 is to be iso-propyl, then it would have been referred to as CH(CH3)CH3 ; when C3H7 is to be propyl, then it have been referred to as CH2CH3CH3. The fact that it's only part-structural formula, means the parts of the molecule that are ambiguous (ie. C3H7) could refer to *either* propyl or iso-propyl. So considering both stereoisomers and structural isomers, there are several different (permutations and combinations of) molecules possible for the part-structural formula given in the question.
1 month left to A levels liao... HUAT AH!!! ;D -
Originally posted by gohby:
Hi UltimaOnline,
Your cogent explanations are amazing - thank you very much. :D
1.
Which of the following will not be produced when 1-chloropropane is heated in ethanolic sodium hydroxide?
CH3CH2CH2ONa
CH3CH2CH2OCH2CH3
Answer: A.
Remarks: How is B produced during the reaction?
2.
http://img.photobucket.com/albums/v700/gohby/Chemistry/compoundy_zpsd5n21kuf.jpg
Remarks: Choice 3 is correct. Is it due to electrophilic substitution with phenylamine, even though the amine is not a primary amine. Students would know that bromine water undergoes electrophilic substitution with phenylamine - would they be required to infer that it would undergo the same reaction even where the amine is not a primary amine?
3.
http://img.photobucket.com/albums/v700/gohby/Chemistry/Cyanohydrin_zpsp7p1b7v6.jpg
Remarks: How do I know if C is correct? How do I know that the cyanohydrin produced does not undergo an SN1 reaction and reacts with ammonia thereafter to form an amine?
4. Re hydrolysis of halogenoalkanes/acyl chlorides/esters/amides (sorry this is long!):
(i) What affects the rate of hydrolysis - the strength of the C-X bond or the extent of δ+ on the C atom?
In alkyl chlorides, the rate of hydrolysis is dependent on the strength of the C-X bond and not the polarity of the C-X bond. However, when we are juxtaposing the rate of hydrolysis between different groups, such as acyl chlorides and (halogenoalkanes, for instance), it is mentioned that acyl chlorides are readily hydrolyzed due to the electron withdrawing O and Cl atoms intensifying the partial positive charge on the C atom.
(ii) How do I compare the rate of hydrolysis across different groups (esters, amides, acyl chlorides, phenoxide, alkoxides, halogenoalkanes)? I know esters and amides hydrolyse slower as compared to acyl chlorides from the conditions required to hydrolyse them, but what is the basis of the different rates of hydrolysis?
Glad to be of help ;)
Q1. SN2 nucleophilic attack by the ethanol solvent molecule (ie. a competing nucleophile).
Q2. Yes, the N atom is sp2 hybridized (bond angle 120 not 107), and the lone pair is delocalized by resonance into the benzene ring, making it a strong activator and an ortho-para director. Hence 2 Br atoms (from 2 moles of aqueous Br2) will be substituted onto the (single available) ortho position, and para position, relative to the N atom. The 3rd mole of Br2(aq) undergoes electrophilic addition with the alkene group.
Q3. Ammonia is a weak nucleophile, and there are no viable leaving groups (both OH- and CN- are more unstable compared to NH3), and hence neither SN1 nor SN2 are possible.
Q4. (i) Both factors simultaneously, of course. Which outweighs which? Depends on context. For alkyl halides, the differing C-X bond strength is the more important factor that determines rate of hydrolysis (ie. going down Group 7). Comparing alkyl halides vs acyl halidies, then the much greater magnitude of partial positive charge on the acyl C atom, predominates to reduce the Ea required, allowing for instantaneous hydrolysis (at room temperature), compared to hydrolysing alkyl halides where heating under reflux is required.
(ii) Comparing hydrolysis rates of esters & amides versus acyl halides :
This is beyond the syllabus, and not all JC teachers understand this well, let alone JC students. So unlikely for Cambridge to ask students to explain, but of course students need to be able to at least state so.
There are 2 reasons for the differing rates of hydrolysis : Resonance within the reactant, and stability of the intermediate products.
As mentioned in my previous post, esters & amides are resonance stabilized ; to be precise the C atom in the resonance hybrid of esters & amides are not as electron deficient (and thus not as electrophilic) compared to aldehydes, ketones and acyl halides. For aldehydes and ketones, it's because there is no adjacent O and N or halogen atom to donate electrons by resonance. For acyl halides, the halogen atom can and does donate electrons by resonance, but not as effectively compared to O or N atoms in esters & amides. Why? Case 1 : F is the most electronegative element in the Universe, so it withdraws electrons by induction even more strongly than it donates by resonance. Case 2 : Cl, Br, I don't withdraw much by induction, but they donate even more poorly by resonance (ie. weak partial double bond character), due to the ineffective sideways overlap between their diffused 3p or 4p or 5p orbitals with the 2p orbital of the C atom. Consequently, the magnitude of partial positive charge on the C atom in acyl halides is (all things considered) still larger, compared to in esters & amides (where the adjacent O or N atom donates electrons by resonance to the C atom, counteracting the electron-withdrawing by resonance effects of the acyl O atom).
Stability of the intermediate products : Notice that while acyl halides can be readily hydrolysed under neutral pH aqueous conditions at room temperature, hydrolysis require both an acidic or alkaline pH, as well as heating under reflux. The higher temperature due to the higher Ea is attributed to both the resonance factor mentioned above, as well as the higher Ea required for the elementary steps in the extended mechanism pathway for hydrolysis of esters & amides (compared to the simple addition-elimination mechanism for acyl halides). At neutral pH, should the nucleophilic acyl substitution occur in a simplistic SN1 or SN2 or addition-elimination mechanism (which it does not), notice that the leaving group, upon elimination has a negative formal and hence ionic charge. Halide ions Cl-, Br- and I- are relatively stable, due to their low anionic charge densities, due to their large ionic radii. But a negative formal charge on a small O or N atom (on the group eliminated during hydrolysis of esters & amides) is strongly destabilizing, due to high anionic charge density on the small O or N atom. Hence an acidic pH is required for proton transfers to lower the Ea for the elimination elementary step. An alkaline pH doesn't lower the Ea for this elementary step, but it does lower the Ea for the preceding elementary step, as OH- (during alkaline hydrolysis) is more electron-rich and thus a stronger nucleophile (thus lower Ea) compared to H2O (during neutral or acidic hydrolysis).
If you're interested, google out (and subsequent to perusing them, draw them out yourself to explain to your students if they're interested and ready) the full curved-arrow electron-flow mechanisms for :
Hydrolysis of acyl halides
Acidic hydrolysis of ester
Alkaline hydrolysis of ester
Acidic hydrolysis of amide
Alkaline hydrolysis of amide
and compare the Ea required for each and all elementary steps, to better understand why acyl halides undergo hydrolysis so much more readily than esters & amides.
PS. btw & fyi, the terms AAC1, AAC2, AAL1, AAL2, BAC1, BAC2, BAL1, BAL2 used for describing the varying acid-catalyzed vs base-promoted, acyl cleavage vs alkyl cleavage, mechanisms for ester & amide hydrolyses, are *not* required and go beyond even H3 Chem level. For H2 & H3 Chem, just regard the hydrolyses mechanisms for esters & amides to be an *extended* addition-elimination mechanism focusing on acyl (rather than alkyl) cleavages, involving a tetrahedral sp3 intermediate, with proton transfers in between.
PPS. btw & fyi, if Cambridge asks for the type of reaction (rather than type of mechanism), hydrolysis of acyl halides, esters & amides are all considered "nucleophilic acyl substitutions" (just as SN1 and SN2 are mechanisms for nucleophilic aliphatic substitutions). So give both "hydrolysis" and "nucleophilic acyl substitution" as the answer. Type of mechanism for the hydrolyses, will be "addition-elimination" (term will suffice for H2 & H3 Chem, but you'll need to draw out the full mechanism for H3 Chem), or if you really feel like showing off, "AAC1, AAC2, AAL1, AAL2, BAC1, BAC2, BAL1, BAL2" for hydrolysis of esters & amides. For generation of esters & amides, type of reaction is still "nucleophilic acyl substitution" but now also "condensation" instead of "hydrolysis". Type of mechanism is still "addition-elimination".
PPPS. btw & fyi, Do your students know the definitional difference between "condensation" vs "dehydration"? Or "hydrolysis" vs "hydration"? (Note that for these 4 terms, there's somewhat different usage between Organic Chem vs Physical Chem contexts). Not many JC students know, and not many JC teachers bother to teach, or teach correctly, on these matters. (Gohby and everyone else reading this post who might be interested, I'll leave it to you to go ahead and explore for yourself these terms, rather than explicitly revealing them on the forum ; if you're a JC student you can try asking your school teacher or private tutor on the definitions and correct usage of these terms, in physical vs organic chemistry contexts *evil laugh*). -
Originally posted by Light5:
Paper:
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_s12_qp_51.pdf
Mark Scheme http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_s12_ms_51.pdf
^Q1.) In the above paper in Q1 part(c) which is an experimental planning question, the examiner asks us to give the range of concentrations appropriate for this experiment. In the mark scheme, he has accepted from 0.8 to 1.39 mol/dm^3 .....but why cant we use a solution with conc less than 0.8 such as 0.5 or 0.2
Furthermore, i am not sure how we will practically make this solution of aqueous copper II sulfate in the lab....we have to ensure each solution is 100 cm^3 in volume but the process is not mentioned in detail in the mark scheme. Please provide me with the necessary steps required to prepare these solutions.
Lastly, in the c(iii) , we are asked to find the molar concentration of ONE of the solutions of copper sulfate but havent we already listed the range of concentrations in part (i)...isnt this part contradictory...if not, please explain how to solve it.
Q2.) I wanted to know from where i can learn about mechanisms in INORGANIC chemistry. How can i predict which reactions will occur and which will not based on the structure, shape,bonding,etc of reactants and stability of products formed....and ultimately how can i deduce the most favorable product that can be formed in a reaction especially when we are asked to "Predict the reaction".
Moreover, how can i understand why certain reactions actually occur,for example by explaing why SO3 + H2O makes H2SO4...which pretty much means learning the mechanisms for the reactions (which i dont know where to learn from)
Also, from where can i read about why certain compounds or elements are present in the way they are for example S8 instead of O2, why SiO2 is giant covalent...i feel these are important because the examiners usually like testing anomalies (of a certain trend ).
Thank You !
I can't help you much with your questions in your post, for a couple of reasons.
The 1st reason is that, assuming you're taking the Nov 2015 A level paper, you're asking all these questions a little too late. Learning allotropic details for elements such as sulfur, or reaction mechanisms (particularly for Inorganic Chemistry), requires going beyond the A level syllabus, and thus requires a lot more time that you currently have. You only have a month left, and (assuming you're a student, not a teacher or private tutor), you should spend the remaining 1 month left revising your within-syllabus content, not trying to learn details and mechanisms beyond the A level syllabus.
The 2nd reason has to do with experimental planning. The mark scheme naturally doesn't give a model answer, but it *does* specify the details required, which you are free to phrase in your own way. Whether you're a student in the UK, Singapore, or some other country taking the CIE exams, your school should have given you Planning notes with sample experiments. If you're think you really need all the marks you can get, including from Planning, then you should buy this book (if it's available locally in the UK or wherever you are, there might still be enough time to buy and study it before your Planning paper).
http://www.worldscientific.com/worldscibooks/10.1142/9565
But my exam-smart advice to all A level Chemistry students (particularly Singapore H2 Chemistry students), is not to worry too much about Planning, or spend too much time preparing for it, because not only is it futile to try to 'spot' or predict the Planning question, the nature of Planning questions and the marks allocated for it, means it's not worthwhile to memorize dozens and dozens of sample Planning experiments.
Skip the Planning question first, complete the rest of the Paper first, then go back to the Planning question and write whatever you can, based on common sense and chemistry sense.
Theoretically, if your chemistry is strong enough to get (almost) full marks for all the theory papers (excluding Planning qn), you can still get your A grade, even if you skipped the Planning qn. So focus on making sure your chemistry is strong, and don't worry about Planning.
Edited : Almost forgot to tell you, for that particular A level exam question you pointed out in the link, you misinterpreted the Cambridge Mark Scheme; it's not that the molarity must be between 0.8M to 1.39M, it's that you must have a range of concentrations over at least 0.8 mol dm–3, which must cover 1.0 mol dm–3, that is to say, you need the maximum molarity to be at least 0.8M higher than the minimum molarity, and that 1.0M must be included. Eg. 0.5M, 0.6M, 0.7M, 0.8M, 0.9M, 1.0M, 1.1M, 1.2M, 1.3M. Notice that 1.3M - 0.5M = 0.8M, which thus satisfies the Mark Scheme requirements.
The 3rd reason has to do with Inorganic Chemistry allotropes. The full reasons for why various elements exist in particular allotropes (ie. varying thermodynamic stabilities and feasibilities under varying conditions), go significantly beyond the A levels, and should not concern you at A levels. Sulfur for instance, has over 30 allotropes. Allotropes of carbon would be more relevant to the A level syllabus, eg. Cambridge could set question on fullerenes and their applications.
If asked about allotropes, Cambridge will certainly give you hints, or at least phrase the question in a way which will be sufficient to enable you to link the questions to various parts of the A level syllabus. For instance, Cambridge *could* ask you to compare and contrast the molecular bond angles and bond strengths between N2 and P4 (since both are elements of Group V), or between CO2 and SIO2 (since both are oxides of Group IV), and *therefore* suggest reasons why nitrogen exists as N2 instead of N4, or why phosphorus exists as P4 instead of P2, or why carbon dioxide exists as simple covalent molecular, or why silicon oxide exists as a giant covalent lattice, etc. Here, the relevant concepts are molecular strain due to angle strain, effectiveness of different electron shell orbital overlaps, preference of forming sigma vs pi bonds, thermodynamic stabilities and feasibilities, etc. Don't worry too much about this, since this isn't in the basic A level syllabus, and thus Cambridge doesn't expect you to memorize such info, but apply your A level knowledge to make reasonable suggestions, with sufficient hints included in the question.
The last reason, (linked to the 1st reason), is that while it is a laudable goal for A level students to learn and apply reaction mechanisms to Inorganic Chemistry (which I do teach to my own BedokFunland JC students), this is something that cannot and should not be learnt at the last minute before the A levels, and certainly not online over a forum. You need personalized guidance for this, and since most school teachers (I assume UK teachers are overworked and underpaid just like in Singapore) won't be willing to teach mechanisms beyond the basic A level syllabus, it is mostly up to private tutors to do so. But it's too late for you to get a private tutor now, 1 month left before the A levels. And if you already had a private tutor, you would be asking him/her, instead of posting these questions here.
Be that as it may, since you asked about SO3 + H2O --> H2SO4, I'll explain the mechanism here :
SO3 is electrophilic or Lewis acidic, and the central S atom has a strong partial positive charge, due to the 3 electronegative, inductively electron-withdrawing O atoms, as well as the 3 single bonds resonance contributor with separation of formal charges (3+ charge on S atom, 1- charge on each O atom), due to ineffective sideways overlap between the 2p orbital of O and 3p orbital of S. In addition, the S atom has vacant, energetically accessible 3d orbitals to accommodate an expanded octet, allowing for the potential for reaction mechanisms with relatively low Ea. Concordantly and consequently, SO3 has the propensity to accept dative bonds, ie. behaving as an electrophile or Lewis acid.
H2O is nucleophilic or Lewis basic, due to the 2:1 ratio of H to O, ie. the magnitude of partial -ve charge on O atom is 2x that of the magnitude of partial +ve charge on H atom. In addition, the O atom has 2 lone pairs. Concordantly and consequently, 1 of the 2 lone pairs on the O atom in H2O is available for donation as a dative covalent bond.
Hence, the hydrolysis of non-metal, covalent, Lewis acidic oxides, generates Bronsted-Lowry acids such as H2SO4.
1st step of mechanism : H2O nucleophile or Lewis base attacks (ie. nucleophilic addition) the SO3 electrophile or Lewis acid. Assuming you used the 3 double bonds resonance contributor, there will be 2 curved arrows : 1 arrow from lone pair of O in H2O, to the S atom in SO3. The 2nd arrow will be from one of the pi bonds, heterolytically cleaving away from S and towards O.
2nd step of mechanism : Intramolecular Bronsted-Lowy acid-base proton transfer reaction. A proton or H+ ion is transferred from the +ve formal charged O atom, to the -ve formal charged O atom. Hence, the curved arrow is from a lone pair on the O- atom to the delta+ H atom, and a 2nd curved arrow from the O-H bond pair cleaving heterolytically towards the O, to get rid of the +ve formal charge.
Concordantly and consequently, you obtain your sulfuric(VI) acid, which is Bronsted-Lowry acidic due to the sulfate(VI) conjugate base stabilized by having its negative formal charges (for either the uninegative or dinegative conjugate bases) delocalized by resonance over 3 or 4 (depending on which conjugate base HSO4- or SO4 2-) electronegative, electron-withdrawing O atoms. -
Originally posted by hoay:
Q. Buckminsterfullerene has the formula C60.
The bonding in buckminsterfullerene is similar to the bonding in graphite.
Which of the following is true?
A All the bond angles in buckminsterfullerene are 120°.
B The melting temperature of buckminsterfullerene is higher than that of graphite.
C There are delocalized electrons in buckminsterfullerene.
D On complete combustion, buckminsterfullerene forms carbon dioxide and water.
It seems that B is the answer.
But D and A are equally right snce both will give CO2 and H2O upon combustion and all Carbon in graphite are sp2-hydridized so the bond angle will be 120....
Alterntively question will be "Which of the following is NOT true"?
Please help.
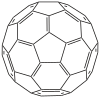
https://en.wikipedia.org/wiki/BuckminsterfullereneThe first thing to note, is that buckminsterfullerene consists of alternating pentagons and hexagons, with 2 different bond lengths and bond angles even in the resonance hybrid (ie. not just in the resonance contributors).
A is wrong because if all C atoms had exactly 120 degrees bond angles, the molecule would be planar (like the graphene layers in graphite), but obviously buckminsterfullerene is spherical, and therefore although the C atoms are considered (largely) sp2 hybridized, the bond angles necessarily deviate from 120 degrees in order to achieve the spherical molecular geometry, which also results in molecular strain due to ring strain due to angle strain. Interestingly, although every C atom in buckminsterfullerene belongs simultaneously to both a pentagon and a hexagon, the ring strains are concentrated in the pentagons, which are non-contiguous and are required for the 'wraparound closure' of the 'graphene sheet' to form the spherical buckminsterfullerene molecule. A mathematical model of the bond angles would be 108 degrees within each pentagon, and 120 degrees within each hexagon. The actual values for the 2 different types of bond angles in the resonance hybrid of buckminsterfullerene would therefore be slightly greater than 108 degrees (within each pentagon), and slightly lesser than 120 degrees (within each hexagon), after taking resonance into consideration.
B is wrong because to melt buckminsterfullerene, you only need to partially overcome the intermolecular van der Waals attractions. To melt graphite, you need to partially overcome *both* the van der Waals attractions between the graphene layers in graphite, as well as the partial double covalent bonds within each graphene layer.
C is correct, as electrons can indeed be delocalized by across the surface of each molecule (not across molecules), due to sideways overlapping of unhybridized p orbitals of each (largely) sp2 C atom. However, the electron delocalization and aromaticity is only partial, and not complete. This is related to option A and the structure (see diagram) above. Buckminsterfullerene tends to avoid having double bonds in the pentagonal rings (ie. in the resonance hybrid, the pentagonal bond lengths and strengths have mostly single bond character, while the hexagonal bond lengths and strengths have greater partial double bond character), which makes electron delocalization poor, and results in buckminsterfullerene being only partially aromatic.
D is wrong because buckminsterfullerene isn't a hydrocarbon, but just carbon (ie. an allotrope of). Hence only product of complete combustion will be CO2 (no H2O).
-
Originally posted by Kahynickel:
Q. Aluminum is the most abundant metal in earth’s crust.
(a)(i) Complete the full electronic configuration of Al atom. Label the energy levels to indicate the principal quantum number and the type of orbital at each energy level
(ii) Sketch the shapes of each of the following sub-shell in Al. Label each sub-shell you draw.
………... ………… ………...
lowest-energy occupied highest-energy occupied highest energy unoccupied
sub-shell sub-shell sub-shell
Ans that i am looking for:
lowest energy occupied.....Is
highest energy occpied .....3px or 3py or 3pz
highest energy unoccupied..... ??
I have confusion about the last one would it be 4s or one of the 3p susb-shell since the next eelctron will go to the any of 3py or 3pz.??
Hi Kahynickel, you're setting this qn for your students?
You should use the term "orbital" rather than "subshell" in the question, since for instance, the 3p subshell consists of the 3px, 3py and 3pz orbitals.
By default, electrons will fill up (what we humans label as) the px orbital, before filling up py and pz orbitals. Of course, this is just a human-thing, but nonetheless, that's what is expected of A level students.
As such, highest energy orbital occupied should be 3px, and highest energy orbitals unoccupied should be 3py and 3pz (accept either drawn).
The 4th electron shell (there's no such thing as "quantum shell", only "quantum number", a common error made by school teachers in Singapore and around the world) is irrelevant to Al (being a period 3 element), and thus orbitals from the 4th (or greater) electron shell, will not be acceptable as an answer. -
Originally posted by BCME:
With reference to JJC_2013_P2 7(d)(iii),
Sorry i know there will be 2 lone pairs but i don't really understand why there will be a ring strain
After each donor N atom has donated the lone pair in the form of a coordinate dative covalent bond unto the vacant orbital of the central metal cation, the electron geometry about the N atom is tetrahedral, ideally with bond angle 109.5 degrees, ie. requiring sp3 orbital hybridization.
This is still possible using ethane-1,2-diamine (American common name ethylenediamine). But with hydrazine, if both N atoms each donate a dative covalent bond unto the central metal cation, you end up with a 3-membered ring, ie. a triangle consisting of the metal atom and the 2 N atoms.
Mathematically, the bond angle within the triangle would be 60 degrees, which deviates significantly from the ideal 109.5 degrees bond angle (ie. angle strain).
Consequently, the destabilizing inter-electron (bond pair - bond pair) repulsions as a result of the angle strain results in the ring being strained, and therefore destabilizes the entire coordination complex, making it thermodynamically unfeasible for hydrazine to function as a bidentate ligand. -
In regard to this article : http://edition.cnn.com/2015/10/05/asia/singapore-smartest-kids/index.html
Many people think Singaporeans are only exam-smart because they blindly memorize and regurgitate. While that may apply somewhat to lower levels (eg. PSLE and O levels), it doesn't apply so much to higher levels, eg. A levels*, Olympiads, University levels. And at all levels, standards continue to increase and bell-curves continue to be steeper, due to increasing competition for academic qualifications and jobs, both intra-nationally and inter-nationally in an increasingly globalized context.
*Singapore A levels are, by some standards and for some subjects, already more difficult than international A levels.
In short, it is overly simplistic and wrong to say Singapore students are exam-smart merely because they blindly memorize. Blind memorization alone is increasingly insufficient for A level papers (eg. H2 Chemistry), and absolutely insufficient for Olympiads and international competitions.
If Singaporeans always top the international rankings in mathematics and science, and are truly intelligent instead of blindly memorizing and regurgitation, why then (critics love to use this argument), has Singapore produced no Nobel prize winners?
One major reason (there are others of course), which appears to have been overlooked by almost everyone else discussing this issue, is the exceedingly pragmatic Singaporean culture when it comes to money and career. (This holds true for other countries around the world as well, but especially for Singapore where costs of living continue to escalate amidst increasing competition from foreign talent and rising rentals by greedy REITS, even as the Sing dollar strengthens against other regional currencies... obviously there are unethical parties, organizations and industries covertly exploiting this, which is where all the money disappears to instead of benefiting the population... but that's the topic for another discussion altogether).
Whilst ideally (and arguably), the most brilliant minds in any population should work and contribute to mankind's scientific advancement, but here in Singapore, other than Medicine, almost all the top students (who could have qualified for scientific careers) prefer the hugely more financially lucrative careers of banking and finance, law and other careers more profitable than in Science. The kiasu-ism and kiasi-ism, fear of not earning enough to survive or provide comfortably for your future family in increasingly competitive Singapore, coupled relentless societal pressure (eg. Hollywood glamorized Wall Street financial exploits, the proliferation of Top 10 Richest in Singapore / Asia / the World on the internet and social media), motivates one's greed to be the elite cream of the crop, the top 5% elite stratum of society right at the of society, in this dog-eat-dog, survival of the fittest, rapidly globalizing Singapore.
In short, this is one of the very real, major reasons, one that has been mostly overlooked and missed, why Singapore hasn't produced a Nobel prize winner. Not that there aren't any Singaporeans intelligent enough to do so, but that most Singaporeans strongly prioritize financial security and career stability over a PhD research career in the Sciences, which pays out a mere pittance of a fraction compared to careers in banking and finance.
Banking and finance doesn't contribute anywhere as much benefit to mankind, as compared to science, medicine and technology; but it's where the money is, and therefore it's where most of the top brains, minds and students in Singapore, choose to go. -
Orbital Radius posted :
I will want to add my insight upon Ultimaonline's view.
Having recently graduated from the Jc level and being a med aspirant,I have clearly observed this alarming mindset of Singaporeans,that is fear of time and money.
I myself,wasn't fortunate to go through the undergraduate route for med and am deciding to embark on the graduate route,that simply means getting a degree before pursuing medical degree.I have talked to a number of ex students from top notch jcs and 80% of them have told me outfront that such a route is time-consuming and a waste of time.
To add on,one senior of mine has also acknowledged that Singaporeans tend to prefer stability over taking risks.
"My friends are already working,yet I am still studying," this comparison is usually centred around the other Nus/ntu undergraduate medicine students.
This is a total opposite to Australia and US medical education.From my readings online,I have came to the attention that our foreign counterparts are more driven towards their goals even if money and time is a factor of consideration.
Have you heard of people over 35 applying for medical school or people with music academical background going for medical school?
Other than med school,I do love to share about the Nobel prize issue,since that's my back-up career(PhD research) if I fail medical entry again,lol
A friend of mine under A star, brought to my attention that the key performance indicator in the working world is a basic requirement for promotion. When I asked the similar question about Singapore's potential for a Nobel prize, He gave a quick grin and highlighted that many of the researchers are only focused on career progression and nothing more than that.They are more than satisfied with their current job and not willing to dedicate additional time and effort to push their research frontiers to the next level.
Ultima Online :
Thanks, Orbital Radius. Totally correct, in the scientific research front, even for the few Singaporeans who choose to go into scientific research (instead of say, finance & banking), it's not that they are not smart enough to be of Nobel prize winning caliber, but rather that they are both self-focused and institute-directed to apply scientific research into profit-driven commercial projects (eg. engineering, pharmaceuticals, etc), registering intellectual property and copyright patents for the institute and sponsors, etc. In other words, Singaporean scientific researchers are only allowed to research what their commercial sponsors want, which are commercialization of profit-driven products and technology. None of these warrant a Nobel prize, which is for contribution towards advancement of mankind's scientific knowledge pushing past current boundaries. No Singaporean scientist will ever win a Nobel prize, *not* because they're not intelligent enough, but simply because that's not where their sponsor's money is directed at (it takes years of money-losing research before one is able to make new discoveries worthy of the Nobel prize, and Singaporeans prioritize financial security and career stability over loss-making, risk-taking Nobel prize winning endeavors). -
Institute / Employer / Corporate Sponsor : "Eh Mr Singaporean Scientist, you better faster give me results on the research I directed you to do, so I can faster get my copyright patent and sell my billion dollar product soon! Don't waste my money researching some useless frontier stuff for that Nobel prize nonsense hor! Or else I'm gonna withdraw your team's funding, or even fire your ass!"
Singaporean Scientist : "I can't afford to get fired, with the costs of living rising in Singapore so steeply year after year, and with all the foreign talent competition coming in year after year. I need to feed my family, that's getting my priorities right. Besides, even if I had tens of millions of dollars of my own money (which I don't) and years of my own time to spend on frontier research to advance mankind's scientific understanding worthy of a Nobel prize, even in the slim chance I succeed (after spending many millions of dollars and many years of my life), there's no way I can easily convert such knowledge (which will be made freely available to all mankind, unlike products and technologies which can be copyrighted) into cash, and if anything, other MNC corporations and pharmaceutical giants are the ones who will eventually greedily profit from my years of Nobel prize winning research, and I get nothing! What the hell! It just doesn't make sense for me to do this. No point. Not worth it. If there's anything being a Singaporean has taught me, it's to be realistic, pragmatic and down-to-earth, prioritizing security and stability over all else. We're certainly as intelligent as scientists from any other country, but we'll leave the Nobel prize winning work to them (scientists who in some countries and institutions are better funded to pursue frontier research, and whose wealthy employers & sponsors passionately believe in contributing to mankind over copyrighting profit-making patents). Not worth it for us." -
Hi Gohby,
Q1. Yes you're right. But in addition, even before LCP has a chance to participate, the fact is the question didn't specify the molarity of the KCl solution. In theory, if significantly higher than 1M (ie. concentrated), Cl- would be oxidized to Cl2 at the anode immediately. If significantly lower than 1M (ie. dilute), then H2O would be oxidized instead. Data Booklet redox potentials are only for standard molarities, ie. all species 1M on both LHS and RHS. In practice (for almost all aqueous solutions, unless super dilute or super concentrated), you'll almost always get a mixture of both O2 and Cl2 generated at the anode.
Q2. You're right for option 3. For option 2, Cr3+ has a high cationic charge density, and thus the hexaaquated complex ion will, just like Al3+(aq), lose protons to the solution, making it acidic, thus protonating carbonate ion, generating carbonic acid, which exists in equilibrium with, and hence can decompose into CO2(g) and H2O(l) with thermodynamically favourable positive entropy change, and also as predicted by Le Chatelier's principle.
You're welcome :)Originally posted by gohby:Hi UltimaOnline,
I have some questions on electrochem:
1: VJC 2012 Prelim P1/Q8
Remarks: Depending on the answer scheme provided by different parties, the answer is either B or C. I would like to confirm if the answer is C. Here are my thoughts: comparing Eox potentials, water is oxidised preferentially to Cl- at the anode. Hence, H+ ions move towards Z, thus turning the colour of the litmus red. However, I would think after a few minutes, when sufficient oxygen has been evolved, owing to LCP, the oxidation potential of water would have been lower than -1.36V, thus chloride ions in the KCl starts to oxidise instead, thus turning the litmus white. Am I right?
2. Which of the following compounds do not exist under aqueous conditions?
1 Cobalt(II) chloride
2 Chromium(III) carbonate
3 Iron(III) iodide
Answer: 2 and 3.
Remarks: For 3 is it because the Ecell for the redox reaction between Fe3+ and that of I- is positive (+0.23V)? For 2 chromium (III) cannot undergo a redox reaction with water as the Ecell is negative - so why can’t it exist? What about 1?Thank you! :)
-
Originally posted by gohby:
Hi UltimaOnline,
I have a couple of questions on inorganic chemistry:
Which of the following is true?
-
BF4- is able to act as a ligand.
-
Aqueous bromine and aqueous chlorine can be distinguished by adding iron (II) sulphate, followed by aqueous sodium hydroxide to separate volumes of aqueous bromine and aqueous chlorine.
-
When AgNO3(aq) is mixed with FeCl2(aq), a grey precipitate is formed, which does not dissolve in NH3(aq).
Answer: 1, 2 and 3
Remarks: For 1, how is BF4- ion able to act as a ligand despite it not having lone pair of electrons? For 2, what happens? I suppose there could be a redox reaction between iron (II) and bromide/chloride ions, but how is this a way to distinguish between the halogens? For 3, what is the chemistry involved in this reaction? Isn’t AgCl of cream colour, which dissolves in ammonia?
2. [DHS Prelim 2012]
S is a transition element. The 3d sub–shell of S in the compound K[S(C2O4)2(NH3)2] contains 3 electrons. How many unpaired electrons does S contain when it is in the elemental state?
A: 2 B: 3 C: 4 D: 5
Answer: C
Remarks: I thought the answer is 6: from the compound, I gather that the charge on S is 3+. Hence, during the ionisation, I would think that 1 4s electron and 2 3d electrons will be taken away. As electronic configuration of S is [Ar]3d54s1 prior to ionisation, wouldn’t there be 6 unpaired electrons in the elemental state of S?
3. http://img.photobucket.com/albums/v700/gohby/Chemistry/trichloro_zpsb0anyptf.jpg
Answer: B
Remarks: I’d like to confirm if my understanding is correct. Firstly, sodium chloride and aqueous bromine will be formed. When I add excess concentrated sodium hydroxide, bromine will disproportionate (assuming that the reaction is carried at temperatures above 15 degrees) to Br- and BrO3-. I am assuming BrO3- to be colourless. As trichloromethane is denser than water it will form a colourless layer below all the ions.
Thank you! :)
Hi Gohby,
Q1. BF4- cannot act as a ligand, but it can function as a source of a F- ligand. (Qn is phrased ambiguously, so qn's fault). If molarities are less than standard molarities, only Cl2, but not Br2, is strong enough an oxidizing agent to oxidize Fe2+ to Fe3+, which you can confirm by the colour of the Fe(OH)3(s). The grey ppt isn't AgCl (which is white, btw), but Ag(s).
Q2. Ignoring the dative bonds from the ligands, since the 3d subshell of the metal ion contains 3 electrons, and it's OS is +3, thus the metal ion's 4s orbital is vacant. Hence reducing the metal ion to it's elemental atomic form, we add 3 electrons. 2 of these electrons will first go into 4s (since we're working backwards ; when ionizing from atom to cation, always remove 4s electrons before adding to 3d), and the 3rd will go into the 3d subshell. This gives a total of 4 electrons (all unpaired) in the 3d subshell.
Q3. You're correct. But just to add, the actual mechanism for all 3 halogens (chlorine bromine iodine), is the halogen first disproportionates into the halide ion and the halonate(I) ion. The halonate(I) ion then further disproportionates into the halide ion and the halonate(V) ion. For chlorine, the disproportionation of chlorate(I) ion occurs only upon heating at 70 deg C. For bromine and iodine, this occurs readily at room temperature (they require much lower Ea for the disproportionation redox reaction). Bromate(I) ion can only be isolated at 0 deg C, while iodate(I) ion can never be isolated at any temperature. Fluorine behaves very differently with NaOH(aq) because it is the king of electronegativity. Although the reaction of fluorine with NaOH(aq) is not in the H2 syllabus (and thus H2 student's needn't know or memorize what happens), but Cambridge may still come up with "suggest suggest" type of challenging questions for this beyond-syllabus reaction.
Welcome :) -
-
Originally posted by gohby:
Hi UltimaOnline,
Many thanks once again for your cogent explanation. :)
For 1, why isn’t Br2 able to oxidise Fe2+ to Fe3+ since the Ecell is +0.30V?
For 2, I don’t understand why wouldn’t the electronic configuration of the element be [Ar]3d�4s¹ instead of [Ar]3d�4s² since a half-filled 3d subshell would afford extra stability. If it were [Ar]3d�4s¹ then there would be 6 unpaired electrons.
5: What is the trend of the pH of aqueous Group II chloride going down the group? Are they all neutral?
6: Which of the following gives the best description of the reactions of Group II metals and their compounds?
-
All Group II oxides undergo neutralisation with hot acids to give a salt and water.
-
Beryllium hydroxide is amphoteric due to the high charge density of the Be²+ ion.
Remarks: The answer is 1 (I agree), but what’s wrong with choice 2? Is it because it does not give the best description of the Group II reactions (since it only talks about beryllium as opposed to Group II hydroxides in general), or is it because choice 2 should be “Beryllium hydroxide is amphoteric because it reacts with acids and bases� → but that begets the question as to why does it even react with acids and bases in the first place.
7: X is a mixture of two compounds. When X is treated with an excess of dilute hydrochloric acid, a colour gas is evolved and some, but not all of the mixture dissolves. Which one of the following mixtures could be X?
A Ba(NO3)2 and Ca(OH)2
B Ag2SO4 and CaCO3
C CaCO3 and MgSO4
D Ca(OH)2 and MgCO3
Remarks: Since not all of the mixture dissolves and silver chloride is insoluble, B is the only option - but what is the colour gas that is evolved? They aren’t referring to carbon dioxide right?
8: ACJC Prelim 2012 P1 Q17
Remarks: I agree with the answer B, but why is D not a possible answer? (I reckon the position of elements down the group would be X -> Z -> Y)
Hi Gohby,
Q1. Under standard molarities, yes (and H2 students must memorize this) both Cl2 and Br2 can oxidize Fe2+ to Fe3+. But since the answer given stated this option was correct (of course, the student can't see the mark scheme, but based on option elimination : A is 1+2+3, B is 1+2, C is 2+3, D is 1 only), therefore it implies the molarities are less than 1M for the halogens (ie. a diluted solution such that only Cl2, but not Br2, is able to oxidize Fe2+ to Fe3+). Even under standard molarities, there may be an observable difference between the amount of Fe(OH)2 vs Fe(OH)3 ppts generated using Cl2 vs Br2. But you're right, this question is problematic.
Q2. Usually for such qns, you'll not be expected to state the electron configuration as 4s1 3d5 unless the element is specifically stated as Cr. Because in most other such MCQs, the element may not be either Cr or Cu (where a tweak is needed). However in this particular MCQ, your argument that the qn implies it is Cr, and therefore having electron configuration 4s1 3d5 (instead of 4s2 3d4) is fair, and as such Cambridge (in a P2 or P3 qn) would accept either answer. However, since this MCQ did not have an option "6 unpaired electrons", therefore the DHS teacher didn't want the student to tweak the electron configuration. But you're right, this question is problematic.
Q5. They tend towards neutral down the Group. Near the top of the group, they are acidic, due to high cationic charge densities, their hexaaquated complex ions have weakened (less polarized) O-H bonds and tend to dissociate protons, making the solution acidic. The counter ion Cl- has low anionic charge density, and so its effect on pH is negligible.
Q6. [Edited 25 Nov 2015] : For the oxide and the hydroxide of any metal to behave as a Bronsted-Lowry base, is obvious, as both the oxide and hydroxide anions have a propensity to accept protons to lower their anionic charge densities to stabilize themselves. Both the oxide and the hydroxide of Be are equally acidic, because the high cationic charge density of Be enables both the oxide and hydroxide to accept 2 *additional* moles of OH-(aq) (the oxide will first undergo hydrolysis to generate 2 OH-(aq), which are already present in the hydroxide ; subsequently for every mole of Be2+, 4 moles of OH-(aq) ligands complex with the Be2+ Lewis acid to generate the ionic tetrahydroxoberyllate(II) coordination complex), thus behaving as a (Lewis, not Bronsted-Lowry) acid.
The reason why I earlier suggested replacing the word "hydroxide" with "oxide" in your statement about amphotericity, is simply because the A Level H2 Chemistry syllabus focuses on the acid-base-amphoteric nature of the oxides, rather than hydroxides. So yes, you're right that both the oxide and the hydroxide of Be are equally amphoteric, but for A levels, it's the oxide that's tested rather than hydroxide, so if it were a P2 or P3 qn, I would still recommend you to write "oxide" rather than "hydroxide". Since we're left with P1, yes indeed, you (all students taking the A levels) should be aware that both the oxide and the hydroxide of Be are equally amphoteric.
Q7. Replace the typo word "color" with "colorless", and you're good to go.
Q8. As you say, D is only *possible*, it is not *confirmed*. And therefore, D is wrong. D implies "confirmed", not "possible". -
-
Originally posted by Mrworry:
How to choose whether Cu2+ will be reduced to Cu+ or Cu in electrochemistry questions?
i.e reaction between KI (aq) and Cu(SO4)2 (aq)?
The standard reduction potential of Cu2+ to Cu is +0.34V, which is more positive compared to the standard reduction potential of Cu2+ to Cu+, which is +0.15V. Hence, always reduce Cu2+ to Cu (especially for electrochemistry questions, since regardless of whether the standard cell potential using Cu2+ to Cu+ reduction half-equation is feasible or not, the standard cell potential using Cu2+ to Cu reduction half-equation will always be more positive and hence more thermodynamically feasible, ie. Gibbs free energy more negative), *unless* otherwise indicated / hinted by the question (eg. in inorganic deductive-elucidation questions), or when it's for Fehling's and Tollen's, in which H2 Chem students are required to memorize and be able to write out the balanced redox equation in which the aldehyde* is oxidized to carboxylate ion, and Cu2+ is reduced to Cu+ in brick-red / brownish-red ppt of Cu2O(s)*.
Update : Turns out MrWorry was asking about the special case reaction to generate copper(I) iodide by mixing an aqueous solution of sodium or potassium iodide and a soluble copper(II) salt such copper sulfate.
Cu2+ + 2I− → CuI2
The CuI2 immediately decomposes to iodine and insoluble copper(I) iodide, releasing I2.
2 CuI2 → 2 CuI + I2
Under standard conditions, the cell potential (ie. reduction potential @ cathode + oxidation potential at anode) for this reaction is negative, ie. Gibbs free energy is positive and hence thermodynamically infeasible. So why does this reaction occur to generate copper(I) iodide?
As it turns out, as a consequence of the CuI(s) precipitation, [Cu+] decreases, shifting the position of equilibrium to the RHS as predicted by Le Chatelier's principle, making the reduction potential of Cu2+ to Cu+ more positive. Concordantly, the cell potential under these non-standard conditions (where CuI(s) precipitates out), becomes positive, and the reaction becomes thermodynamically feasible.
Further Update :Originally posted by Sugarfortress :Hi Ultima, pertaining to the above concern, how do we reconcile the possibility of the precipitation of insoluble CuI also lowering [I-] and thus shifting the position of eqm of I2/I- reduction equation to the right hence making it more positive and in that sense still make the cell potential for the reaction negative?
Thanks so much!
Excellent point, Sugarfortress. This *appears* to be irreconcilable at A levels, given the stoichiometry and magnitudes of the relevant oxidation and reduction potentials. A easy workaround would be to suggest that excess I- be required (again, non-standard molarities). However, as it turns out, there's actually a more important reason that enables the redox reaction to proceed to generate CuI(s) even without excess I-. Which is, the reduction potential of Cu2+(aq) to Cu+(s) precipitate is actually far more positive than given in the Data Booklet, which is for aqueous species, ie. Cu2+(aq) to Cu+(aq). So much so that as CuI(s) precipitates out, the magnitude of the decrease in oxidation potential of I- to I2, is still far outweighed by the magnitude of increase in reduction potential of Cu2+(aq) to Cu+(aq) to Cu+(s). As such, admittedly my earlier explanation is simplified and incomplete, but would still suffice for A levels, should Cambridge ever ask this question.
--------------------------------------------------------------------------------------
* The following are exceptions (not taught in Singapore JCs to H2 Chem students) for Fehling's and Tollen's :
Methanal is (the only aldehyde to be) able to reduce the Cu2+ in Fehling's solution to either Cu+ or Cu, and hence (depending on factors such as molarity) you may get either a red ppt of CuO(s), or a copper mirror of Cu(s), or both.
Methanoic acid is (the only carboxylic acid to be) able to react and give a positive result with both Fehling's and Tollen's. This is because the oxidation potential of methanoic acid to carbonic(IV) acid is large and positive, concordant to the observation that H2CO3(aq) exists in equilibrium with, and hence can decompose into, CO2(g) and H2O(l) with thermodynamically favorable positive entropy change, and also as predicted by Le Chatelier's principle since CO2(g) leaves the reaction mixture, pulling the position of equilibrium over to the RHS.
Ketones do not react with Fehling's and Tollen's. Alpha-(pri)hydroxy-ketones are (the only ketones to be) able to react and give a positive result with both Fehling's and Tollen's.
(Bonus challenge : Draw the curved-arrow electron-flow mechanism in which the alpha-(pri)hydroxy-ketone prototropically tautomerizes first to an ene-diol, then further prototropically tautomerizes to a alpha-(sec)hydroxy-aldehyde ; the redox reaction with Fehling's and Tollen's pulls the position of equilibrium to the RHS, as predicted by Le Chatelier's principle).
Benzaldehyde and its derivatives only give a positive result for Tollen's, but not Fehling's. There are 2 reasons for this :
Firstly, the more alkaline Fehling's solution results in benzaldehyde undergoing the Cannizzaro disproportionation reaction instead (which occurs for aldehydes unable to undergo the aldol reaction due to lack of an enolizable acidic alpha proton, such as in benzaldehye ; however in other aldehydes (ie. those with an enolizable acidic alpha proton), the redox reaction with the oxidizing (and tartarate and citrate ligand coordination complexed to prevent Cu(OH)2 precipitation in Fehling's and Benedict's respectively) Cu2+ ion takes thermodynamic (but not kinetic, as heating is required) priority over Aldol reactions, and as predicted by Le Chatelier's principle since the redox reaction is largely irreversible, while the Aldol reactions involve reversible steps. The Aldol reaction may still occur as a minor side-reaction, but does not affect the observable generation of the red Cu2O ppt).
Secondly, using my BedokFunland JC formula "Oxidation State = Formal Charge + Electronegativity consideration", notice that while the oxidation state (OS) of the aldehyde C atom in aliphatic aldehydes is +1, the OS of the benzaldehyde C atom in the original resonance contributor is +1, while in all other resonance contributors (with pi electrons within the benzene ring withdrawn out towards the electron-withdrawing carbonyl oxygen) is 0, and thus the OS of the benzaldehyde C atom in the resonance hybrid is much closer to 0 than +1. As such, it requires a greater magnitude of thermodynamic Gibbs free energy, and thus a stronger oxidizing agent (Tollen's is a stronger oxidizing agent compared to Fehling's, as the standard reduction potential of Ag+ to Ag is +0.80V, which is more positive compared to the standard reduction potential of Cu2+ to Cu, which is +0.34V, even as the redox potentials are tweaked in the presence of the ammonia and tartarate ligands in Tollen's and Fehling's respectively) to oxidize benzaldehyde (OS of carbonyl C atom = 0) to benzoic acid (OS of acyl C atom = +3). -
Originally posted by BCML:
TYS 2010/P1/33
why is option 3 correct? Like how will osmosis increase entropy?
Analogy :
2 rooms, linked by only 1 door (the partially permeable membrane).
Room A only got girls (solute).
Room B only got guys (solvent).
Both rooms (ie. the system) have low entropy.
But now, if some guys from room B with higher guy (solvent) potential crosses the door (partially permeable membrane) to room A with the lower guy (solvent) potential, there will be in increase in disorderliness in room A (coz now got mix of guys and girls), and thus there is a positive entropy change in both room A and the overall system. -
Originally posted by BCME:
TYS2014/P3/5
Why is H2+Ni @150degrees able to reduce the benzene ring but not the amine and carboxylic acid?
Is there any decarboxylation reaction in the syllabus we need to know? Like some sodalime reaction to decarboylate a carboxylic acid
H2+Ni @150degrees is able to reduce the benzene ring *as well as* the nitrile group, but not the carboxylic acid.
First of all, although hydrogenation/reduction of the benzene ring is not in the H2 syllabus, but Cambridge *did* give you the Mr of both Z and Omega, which will enable you to deduce the hydrogenation/reduction of the benzene ring.
Next, in the H2 syllabus you ought to have memorized that heating with H2 gas over Ni catalyst at 140 deg C can reduce nitrile to amine (CS Toh pg 244). And also in the H2 syllabus you ought to have memorized that under no circumstances can H2 gas reduce carboxylic acid to aldehyde or primary alcohol. LiAlH4 (in dry ether) is required for that.
Finally, both hydrogenation of the benzene ring to cyclohexane and hydrogenation of nitrile to amine is easier to achieve using H2 gas, compared to reduction of carboxylic acid using H2 gas (which cannot be done), because the former 2 are simpler addition reactions (ie. adding H2(g) to reactant to get the product), while the latter involves not just addition of H, but also requires elimination of an O atom, which requires chemical reagents (to carry away the O) in a solvated environment (ie. not just gaseous H2).
Decarboxylations aren't in the H2 syllabus, but Cambridge can (and have, in recent years) give you sufficient info in the question to deduce that decarboxylation has occurred.
Two notable decarboxylation reactions that are useful for A level students to be familiar with, are the use of soda lime, and heating beta-keto-carboxylic acids (Singapore-Cambridge H2 Chemistry A Level 2012 Paper 3 Qn 5d).
As a challenge for H2 Chem students, try drawing out the decarboxylation mechanisms for both reactions described above.
Additional decarboxylation reactions that Cambridge can use in data-based questions, are the Hunsdiecker reaction and the Kochi reaction. -
Originally posted by BCML:
TYS 2014/P3/4(d)(i)
Is it possible for the answer to be acid base reaction of the phenol in tyrosine and the compound in (i)? And also if there is acid base reaction, will an ionic bond be formed since there will be an anion and a cation?
TYS 2014/P3/4(b)(i)
Can use LiAlH4 to reduce followed by protonation?
TYS2010/P2/5
Is imine group considered and amino acid? also what is the name of the bond formed? Like can is be considered an amide or peptide bond?
TYS 2014/P3/4(d)(i)
No, "acid-base" reaction is not accepted, because the question is asking for an "interaction" not "reaction" and furthermore the question defines the "interactions" as that responsible for the tertiary structure of the protein.
Yes, the required answer is ionic interaction with the aspartate ion (deprotonated conjugate base form at pH 7).
TYS 2014/P3/4(b)(i)
Cannot, coz -CN would be reduced to -NH2.
TYS2010/P2/5
No, an imino carboxylic acid is an imino carboxylic acid, not an amino carboxylic acid. And no, imino carboxylic acids do not form peptide bonds or groups, as an imine group is significantly less nucleophilic compared to an amine group, due to the lone pair residing in a sp2 hybridized orbital, which has greater % s-orbital character, compared to the sp3 hybridized orbital of an amine group (assuming lone pair isn't delocalized by resonance).
Note however, that for this question, Cambridge is loosely (and obsoletely and arguably erroneously) including a secondary amine group in the definition of an "imine group". A secondary amine group, is after all, still an amine group (ie. lone pair residing in an sp3 hybridized orbital, assuming lone pair isn't delocalized by resonance). As such, it is sufficiently nucleophilic to generate the peptide group, and so, yes in this context (as per Cambridge's loose, obsolete and erroneous definition of an imino acid), the so-called imino acid does form the peptide group and peptide bond, in the protein.
In biochemistry contexts, real or actual alpha-imino acids (ie. properly defined as carboxylic acids with the C=N imine group on the alpha C atom, rather than Cambridge's secondary amine group trying to pass of as an imine group) involved in protein synthesis, are usually metabolic intermediates converted from one particular proteinogenic amino acid, catalyzed by a specific enzyme, and subsequently converted into another particular proteinogenic amino acid, catalyzed by another specific enzyme.