BedokFunland JC's A Level H2 Chemistry Qns (Part 2)
-
Originally posted by Flying grenade:
Topic : atomic radius
Hi! This is from singapore cambridge nov a level exam year 2010,
Paper 1 qn 1
Qn: which element has the largest atomic radius?
Br
K
Kr
Sc
So suggested answer is potassium, as the valence electrons of the atom of K experience the weakest nuclear charge effect among the 4 options above
And Kr is the atom which valence electrons experience the strongest attraction to the nucleus, as nuclear charge effect increases across the same period while shielding effect remain approximately constant across the same period, hence effective nuclear charge increases across the period
My question is , why not Kr ?
From Data booklet :
Atomic radii /nm :
Na : 0.186
Ar : 0.192
Why cant we extrapolate this observation and apply it for period 4?
From Wikipedia :
In order of atomic radius, covalent radius, van da waals radius :
Na : empirical: 186 pm, 166±9 pm, 227 pm
K : empirical: 227 pm, 203±12 pm, 275 pm
In order of covalent radius, van da waals radius :
Ar : 106±10 pm, 188 pm
Kr : 116±4 pm, 202 pm
Thank you (:
https://en.m.wikipedia.org/wiki/Sodium
https://en.m.wikipedia.org/wiki/Potassium
https://en.m.wikipedia.org/wiki/Argon
https://en.m.wikipedia.org/wiki/Krypton
It's not a fair comparison, because K (being a metal) doesn't have van der Waals radius, while Kr (being unreactive) *only* has van der Waals radius. You could say it's Cambridge's fault for setting this problematic question, but for A level purposes, since in the H2 syllabus, you focus only on the concept of increasing electronegativity and ionization energy across a period, instead of the different types of atomic radii (specifically covalent radius versus van da waals radius) which isn't really in the H2 syllabus, thus Cambridge (reasonably) expects you to just apply the concept of increasing electronegativity and ionization energy across a period, and that's it. Don't need to care about covalent radius versus van der Waals.
Other than atomic radius, Cambridge can test you on comparing the *ionic* radii of iso-electronic species, eg. K+ vs Cl- (both isoelectronic with Ar atom). That's about it. No covalent vs van der Waals radius. -
Originally posted by Metanoia:
Hi all, would like to check on an MCQ from 2013.
Which gases that go into the bottom of the blast furnace undergo a reaction during the extraction of iron?
1. Carbon dioxide 2. Nitrogen 3. Oxygen
A. 1 only B. 1, 2 and 3 C. 1 and 3 only D. 3 only
Would you include nitrogen (forming nitrogen oxides) as your answer?
Again, it's Cambridge's fault for setting ambiguous qns like these (every year there'll be a couple of such qns, in which different school teachers will disagree with each other and/or with Cambridge, and teach their students different answers) at both O levels and (especially) A levels.
The most correct answer (regardless of whether it's the answer Cambridge wanted, which is always debatable for such qns), is that all 3 gases do indeed react at the high temperatures present in blast furnances (whether for extraction of iron from its ore, or other purposes).
As nitrogen (the most abundant gas in the atmosphere) is inevitably present in the air used by blast furnaces (air is afterall, the cheapest source of oxygen, and obviously all industries are profit-oriented and want to make as much money as possible by reducing costs), at such high temperatures of blast furnaces, it's inevitable that various oxides of nitrogen (NOx) are generated. Such oxides of nitrogen are toxic to humans, and are known to be an industrial medical health hazard for blast furnace workers.
While the O level syllabus doesn't specifically include mention of this health hazard suffered by blast furnace workers, nonetheless (assuming you believe Cambridge wants you to including nitrogen as the correct answer) at O levels and A levels, students are arguably expected to put 2 and 2 together (presence of nitrogen and oxygen, and at high temperatures) and conclude oxides of nitrogen may (and are) being produced in blast furnaces, albeit as undesirable (and medically hazardous) side-products.
Originally posted by Metanoia:Yup, I'm a tutor and I raised this question to check the consensus; its one of those that could be argued either way.
Appreciated Jurongresident's detailed reply, apologies if he spent the extra time to do it thinking I'm a student.
For what it's worth, the publishers (cambridge?) suggested answer is only carbon dioxide and oxygen, but I thought a student shouldn't be faulted to include nitrogen also.
Accounts of the need for NOX control in blast furnace has been documented, within the secondary textbook; we might also link it to the chapter on Air and Air pollution.
Alas, its one of those ambiguous questions for tutors and students to take note.
TYS publishers are not certainly not Cambridge. TYS publishers pay MOE for the right to publish the questions (the answers are by the various authors hired by the publishers), as the copyrights are co-owned by Cambridge and MOE. The official Cambridge mark schemes are (by law, due to a legal agreement between MOE and Cambridge) confidential and cannot be revealed to the public. It is fairly common (especially for A levels, when it becomes even more complicated) for all the different TYS publications available on the market to all give different answers for an ambiguous, challenging or problematic, open-ended Singapore A level exam question (every year there's a couple of such qns). This happens even for MCQs, but naturally to a lesser extent. -
Originally posted by BCML:
When calculations on TM are done, when do we include the hydrated H2O or the anhydrous salt? Eg 2013 P2 Qn 2(c)(iii) we use the Mr of anhydrous salt
2013 Paper 2
Q3(a)(i) can reagent A be Na metal instead of NaOH?
Q4(a)(i) When we compare between main group and TM can we use NC increase across the group and SE remains relatively constant to compare ENC and from there explain the atomic radii? Since all the elements are in the same period?2013 Paper 3
Q1(c)(i) When we draw the mechanism do we use equilibrium arrow? Sincs that is given in the question?2012 Paper 2
Q2(a)(ii) Can we say that nitrogen containing hydrocarbon is combusted to produce NO gas?
Q2(c)(i) How do we know that delta G is supposed to be measured in joules?
Q5(a)(ii) Do we have to describe the mechanism by steps like nucleophilic attack and regeneration of catalyst in the mechanism?
2013 P2 Qn 2(c)(iii) - If the question doesn't specify "hydrated", you've to use the molar mass of the anhydrous form.
2013 Paper 2 Q3(a)(i) - You've only a small chance that Cambridge might show you mercy and give you that mark, for 3 reasons. 1stly, Cambridge is testing to see if the student knows that phenol, being more acidic than alcohols, can be deprotonated just by the use of NaOH(aq) alone. 2ndly, NaOH is a lot cheaper and less hazardous than Na. You should always only use the cheaper and safer reagent, if both reagents have the same effect. 3rdly, beyond the H2 syllabus, Na being such a strong reducing agent, can actually (under certain circumstances or with certain co-reagents) reduce the benzene ring (eg. Birch reduction).
Q4(a)(i) - Acronyms are not acceptable by Cambridge in the A levels. Don't over-use them even in your own self-revision. No, your answer is relevant only for period 2 and 3 elements excluding d block metals. For this question, you need to specify that 3d orbitals (being more diffused) provide relatively poor shielding for the 4s orbitals electrons, which are consequently more strongly electrostatically attracted to the positively charged nucleus, of which Fe and Cu have 6 and 9 more protons compared to Ca.
2013 Paper 3 Q1(c)(i) - Cambridge won't give a damn either way.
2012 Paper 2 Q2(a)(ii) - You won't get any marks for your answer. Because in the 1st place by definition of hydrocarbon, there is no nitrogen present (immediately zero marks for this part of the question liao). Next, even if some organic fuels contained nitrogen (eg. amine group, nitrile group, amide group, imine group, etc), that isn't the main mechanism (which Cambridge is testing you for) by which oxides of nitrogen are formed. It's the atmospheric N2 which reacts with atmospheric O2 at high temperatures that is being tested here. Be sure you mention the strong NN triple bond, the high activation energy involved, and the high temperatures present in combustion engines.
Q2(c)(i) - If before today you didn't know, after today you know liao. Yes you're supposed to have memorized this in the H2 syllabus lah!
Q5(a)(ii) - As long as you draw out the full mechanism correctly (yes including regeneration of the catalyst), you don't need to describe anything in words. -
Originally posted by Flying grenade:
A level 2014 paper 1 qn 29
Isnt treating a c=c double bond with aq bromine will result it to be a halohydrin ( a br bonded to one C, OH bonded to other C).
Treat Br to the phenol group will result it attached to the ortho position
So wouldn't the max number be 3 br atoms?
If diluted soln, then 3. If concentrated soln, maximum 4 (Br2 added across double bond) is possible. Cambridge knew many students would think as you did, hence they deliberately (and too kindly) left out 3 as a possible option.
Originally posted by Flying grenade:But qn state it is aqueous bromine, not concentrated
Then, can i form dihalide from c=c by using conc br2, instead of X2 in ccl4, dark
Aqueous can be concentrated aqueous. Different from liquid. Yes you can obtain some dihalide (previous qn stated *maximum possible*, which could be a minor product, not necessarily major), but it won't be 100% yield, so you won't get marks for using this method. You should still specify X2 in CCl4 when asked. -
Originally posted by Flying grenade:
A level 2009 paper 2 qn 2g)
How do we know this zn2+ +2oh -> zinc hydroxide ?, ( cant find this in cs toh study guide): )
And why cant or can, instead write this hydrated [Zn(H2O)6]2+ form?
Do we need to know/memorise strength of ligands so that we would know which ligand displace which ligand?
E.g. Zn (OH)2 + NH3 -> [Zn (NH3)4 ]2+ + OH-
(Sorry didnt balance and didnt write state symbol )
Is the NH3 ligand always stronger than H2O ligand? As it displaces H2O ligand from cu2+ complex
In absence of OH-(aq), you write the hydrated [Zn(H2O)6]2+ form. With OH-(aq), you get Zn(OH)2(s) ppt.
No need to memorize ligand strength or ligand field strength (google if you don't know the difference). Just apply common chemistry sense (eg. ligand strength, nucleophilic strength and Bronsted-Lowry basicity strength, all parallel each other because all 3 are functions of Lews basicity strength, with some exceptions you needn't care about at A levels), and most importantly, follow the clues given in the question. If there's a colour change upon adding a reagent, it implies ligand substitution aka ligand exchange has occurred.
On a related note, whether the coordination complex generated has 4 or 6 ligands, is a complex matter (pun intended), and the underlying reasons cannot be fully covered at A levels. For A level purposes, try to familiarize (no need to blindly memorize 100+ coordination complexes, their geometries and colours, etc) yourself with the dozen or so complexes more commonly encountered at A levels. For this purpose, Chang Kim Seng & Jeanne Tan's book, and George Chong's book, will be more useful than CS Toh's book (which is summarized lecture notes). Your own JC should have given you a list as well.
Tips : if the ligands are small and neutral, eg, H2O ligands, there will usually be 6 ligands, ie. octahedral geometry. If the ligands are large, eg. Cl ligands, there will usually be only 4 ligands, ie. tetrahedral geometry, due to steric hindrance or van der Waals repulsions. If the ligands are small but have high anionic charge densities, eg. OH- ligands, there will usually be only 4 ligands, ie. tetrahedral geometry, due to anionic electrostatic repulsions.
The notable special case (which the H2 syllabus specifies you need to memorize), is the deep-blue coloured and with square planar geometry, tetraamminecopper(II) ionic coordination complex, [Cu(NH3)4]2+. The reasons why Cu2+ only accepts 4 NH3 ligands, and why the ionic complex's geometry is square planar instead of tetrahedral, is because of the Jahn–Teller effect and other reasons too complex to be dealt with at A level purposes. For A level purposes, other than the few commonly encountered coordination complexes you need to memorize (their colours and geometries), simply follow the clues which will be provided by Cambridge in the question.
Yes, NH3 ligand is stronger than H2O ligand, but don't forget molarity (ie. concentration) also determines the position of equilibrium in the Kstab or Kf equation (google it), and therefore determines which ligands substitute or exchange away which ligands. In other words, a weaker ligand with a higher molarity, can substitute away a stronger ligand with a lower molarity. Which is why the Cambridge question will specify, "added in excess" to ensure the ligand substitution occurs, due to the molarity affecting the position of equilibrium. -
Originally posted by Flying grenade:
What affects Nucleophilic and electrophilic strength?
Is it How e- deficient an ephile is, and how delta + positive charge on the nucleophile?
Does the electronegativity of an electrophile play part too?
Nucleophilicity and Electrophilicity is affected by several factors :
- Magnitude of charges and charge densities, whether formal charges or partial charges, which is affected by electronegativity (eg. is the negative formal charge on a C atom or O atom?), % s orbital character in hybridized orbital (affecting charge density), atomic/ionic radius, shielding effect, induction and resonance.
- Availability of vacant orbitals on electrophile (addition-elimination mechanism requires lower Ea than SN1 & SN2 aliphatic nucleophilic substitutions, which is one reason why in Periodicity of H2 syllabus, SiCl4 readily undergoes hydrolysis, while CCl4 is hydrolysis resistant; the availability of 3d orbitals in Si (but not C) creates the potential for addition-elimination hydrolysis mechanisms with lower activation energy).
- Availability of lone pair on nucleophile, which is affected by electronegativity, % s orbital character in hybridized orbital (affecting lone pair availability), atomic/ionic radius, shielding effect, induction and resonance.
- Steric hindrance by bulky groups (either on electrophile reducing electrophilicity, or on nucleophile reducing nucleophilicity and increasing Bronsted-Lowry basicity instead) around the electrophilic or nucleophilic atom.
- Viability and stability of leaving group upon elimination (in nucleophilic aliphatic and acyl substitutions ; for electrophilic aromatic substitutions the incoming electrophilic substitutes away a proton, to restore aromaticity and resonance stabilization energy), which is affected by electronegativity (eg. is the negative formal charge on a C atom or O atom?), % s orbital character in hybridized orbital (affecting stabilization of charge), atomic/ionic radius, shielding effect, induction and resonance.
- Endothermicity of bond dissociation enthalpy and its activation energy, or (in other words) the strength of the covalent bond required to be cleaved (be sure to quote Data Booklet values whenever relevant), which is in turn affected by % s orbital character in hybridized orbital (affecting strength of covalent bond, eg. sp2-sp3 bonds stronger than sp3-sp3 bonds), atomic/ionic radius and period of element (effectiveness of overlap, whether head-on for sigma bonds or side-on for pi bonds, is greatest between 2nd electron shell orbitals and other 2nd electron shell orbitals, and much less effective between 2nd and 3rd/4th/5th electron shell orbitals, or 3rd/4th/5th electron shell orbitals and other 3rd/4th/5th electron shell orbitals, regardless of whether hybridized or not), electronegavity & induction (increasing polarity of covalent bond increases it's ionic character and thus strengthens it, which is why CO triple bond is stronger than NN triple bond in Chemical Bonding, or why Al3+ and Mg2+ salts are acidic in Periodicity and Acid-base Equilibria, due to decreasing polarity and thus weakening of O-H bond in water ligands) and resonance (the C-X bond in aryl & vinyl halides have partial double-bond character in their resonance hybrid, hence aryl & vinyl halides are resistant to hydrolysis).
- % s orbital vs p orbital character of hybridized orbitals, as mentioned above, affects availability of lone pair on Lewis base (ie. nucleophile and/or Bronsted-Lowry base; eg. why amines are more nucleophilic & basic over imines, and imines over nitriles; for amides the resonance factor takes precedence), affects strength of covalent bond required to be cleaved (and hence activaiton energy required), and affects stability of charge (both in nucleophile / Bronsted-Lowry base, as well as in the leaving group after elimination). -
Originally posted by Flying grenade:
That 2009 qn,
So the ammonia ligand replaced oh ligand?
So zinc hydroxide is soluble?
Probably sparingly?
From 2009 paper 2 qn 2g - Eh jialat lah, you really don't understand coordination complex equilibria, izzit? You made 2 errors right here : 1st of all, in this question OH- isn't a ligand at all. It's a counter ion which precipitates out Zn2+ as Zn(OH)2(s). *If* you had added excess NaOH(aq), giving a sufficiently high molarity of OH-, then the excess OH- can start to function as ligands, and that's how you get the tetrahydroxozincate(II) coordination complex.
But in this qn, you did *not* add excess NaOH(aq), rather you added excess NH3(aq), giving a sufficiently high molarity of NH3, then the excess NH3 can start to function as ligands, and that's how you get the tetraamminezinc(II) coordination complex.
All 'insoluble' salts are actually 'sparingly soluble'. But most importantly, you need to be able to explain to Cambridge *why and how* the 'sparingly soluble' metal hydroxide solid precipitate, eg. Cu(OH)2(s) dissolves when adding in excess a solution containing a suitable ligand, eg. NH3(aq), in terms of shifting of positions of equilibria across 2 (or more) reaction equations.
Eqn 1 : NH3(aq) + H2O(l) ---> NH4+(aq) + OH-(aq)
Eqn 2 : Cu2+(aq) + 2OH-(aq) ---> Cu(OH)2(s)
Eqn 3 : Cu2+(aq) + 4NH3(aq) ---> [Cu(NH3)4]2+(aq)
When ammonia solution is added to copper(II) sulfate solution, the hydrolysis of ammonia (Eqn 1) generates OH-(aq), and when Qsp (aka Ionic Product) > Ksp (aka Solubility Product), position of equilibrium in Eqn 2 shifts to the RHS, generating Cu(OH)2(s) precipitate. When excess ammonia solution is added, the position of equilibrium in Eqn 3 shifts to the RHS, resulting in a decrease in molarity of Cu2+(aq), which causes the position of equilibrium in Eqn 2 to shift to the LHS, which causes the copper(II) hydroxide precipitate to dissolve. -
Originally posted by Flying grenade:
What is the distinction between equilibrium shifts (to a side ) and lies (to a side) ??
Is it that for *shift* ,
Eqm alr shifted?
While *lies* to a side is the eqm is more inclined towards that side?
When to use which term ?? ):
If you didn't change conditions, and Qc < Kc, then you say position of equilibrium LIES to the RHS. If you didn't change conditions, and Qc > Kc, then you say position of equilibrium LIES to the LHS.
If at first Qc = Kc, ie. system is at equilibrium, but then you changed experimental conditions, then you say position of equilibrium SHIFTED to either RHS or LHS.
If you increased or deceased the moles or molarities or partial pressures of reactants or products, then it's Qc which changes, not Kc.
If you or increased or decreased temperature, then it's Kc which changes, not Qc.
If Qc now < Kc, then you say position of equilibrium has SHIFTED to the RHS.
If Qc now > Kc, then you say position of equilibrium has SHIFTED to the LHS.
All these your own JC never teach you meh? That proves that you (yo JC students reading this) should come for my BedokFunland JC tuition. *hmmph* -
On dissolving silver halide precipitates when adding NH3(aq)
Eqn 1 - Ag+(aq) + Cl-(aq) --> AgCl(s)
Eqn 2 - Ag+(aq) + 2 NH3(aq) ---> [Ag(NH3)2]+(aq)
When you add excess NH3(aq), position of equilibrium for Eqn2 shifts to RHS, hence position of equilibrium for Eqn1 shifts to LHS, hence AgCl(s) halide precipitate dissolves.
With Br-, you need concentrated NH3(aq) because the Ksp for AgBr is much smaller, ie. less soluble. With I-, the Ksp for AgI is so damn small, that no amount of concentrated NH3(aq) or even NH3(l) can dissolve the AgI(s) halide precipitate.
As for why the solubility of AgX decreases down the group, read Jim Clarke's related article on solubility of Group II hydroxides vs carbonates / sulfates :
http://www.chemguide.co.uk/inorganic/group2/problems.html -
Originally posted by Flying grenade:
2014 P3 Q4
I still dont get it. Why only coo- group of aspartic acid can interact with the amino acid di) residue given by the qn
Val and tyr aren't polypeptide chains, their discrete amino acids
Aren't the groups on val and tyr considered R groups?
Aspartic acid's R group is a COO- group. Valine's R group is an alkyl group. Tyrosine's R group is a phenol group. The question isn't asking for interactions between discrete amino acids. It's asking for interactions between R groups of these amino acid residues within a polypeptide chain. Don't lose your marks carelessly like this, it'll cost you at least 1 grade (with steep bell-curves).
------------------------------------------------------------------
Originally posted by Flying grenade:Update
Is my understanding correct, and can help improve my explanation?
Sian, think question interpretation might also be a problem
Is it because :in this qn, the amino acids are part of a polypeptide chain, e.g. val-tyr-asp- etc etc
Then, both val and tyr's nh2 and cooh is used up in amide bond linkage
Only aspartic acid has the extra cooh group, hence can interact with the qn?
But then wouldnt it be Hbond interactions?
I thought of another :
Is it because ph 7, so means its in water? ( thats why the acid and basic group will deprotonate and protonate??)
Thats why will have ionic interactions instead of h bonding?
But if its in water, then hydrolysis would occur, then wouldnt the nh2 and cooh group on val and tyr able to interact? ?
Help!
1st of all, hydrolysis only occurs partially, it's an acid-base *equilibrium* remember?
2ndly, technically (Singapore JCs don't teach this well so it's not surprising JC students don't really understand this), while COOH and NH2 groups are only capable of (both donating and accepting) H bonds, COO- and NH3+ groups are actually capable of participating in both H bonds (COO- can only accept H bonds, while NH3+ can only donate H bonds) as well as ionic bonds, depending on who the other party is.
And yes, Cambridge expects you to automatically first deprotonate COOH groups at pH7, and protonate NH2 groups at pH7.
------------------------------------------------------------------
Originally posted by Flying grenade:If ph7 in water, will it cause the polypeptide chain to undergo hydrolysis and cause it to break into small portions of amino acid residues?
Oi!!! Wah biangz eh, if at pH7 and room temperature, proteins spontaneously "break into small portions of amino acid residues", then you won't be able to remain in 1 piece typing this post with your phone liao (you won't even be able to hold your phone), you'll disintegrate instantly into a sludge. Sludge man!
------------------------------------------------------------------
Originally posted by Flying grenade:Can tyrosine's phenol group interact with nh3+?
If cannot, is it because phenol doesn't ionise in water?
Hydrogen bonding is possible, between the partial positive H atom of NH3+ (the H bond donor), and the partial negative O atom of phenol (the H bond acceptor).
Cambridge already stated in the question, regarding extent of deprotonation of phenol at pH 7. Read the question carefully lah! -
Originally posted by Flying grenade:Wtf sia, then why we learn polypeptide linkages and proteins undergo hydrolysis and break into smaller portions? And thought ph7 the acid and basic groups ionise?
You need to either heat under reflux or use biological enzymes, plus at strongly acidic or strongly basic pH lah. That's how your strongly acidic stomach digests the meat you eat, with proteases enzymes.
Acidic COOH groups and basic NH2 groups do ionize via hydrolysis to generate COO- groups and NH3+ groups.
Different from hydrolysis of the protein's peptide bond within the amide group in the polypeptide chain of a protein (the word "peptide" refers to *either* the peptide bond within the amide group in a polypeptide chain of a protein, *or* the amino acid residues in a polypeptide chain of a protein). Because the amide group within a polypeptide chain bonds two amino acid residues or 'peptides' together, the amide group in proteins is called the peptide group, and the C-N bond within the amide group is called the peptide bond, linking two 'peptides' or amino acid residues together.
Hydrolysis means "chemical reaction with water" (where chemical reactions involve breaking or forming of sigma bonds, while resonance involves breaking or forming of pi bonds only), while hydration means "physical interaction with water" (physical, inorganic & organic chemistry) or "addition of water" (in some organic chemistry contexts, eg. hydration of alkenes to alcohols). -
Originally posted by Flying grenade:
Pcl5 reacts with acid to form acyl chloride
Reacts with alcohol to from halogenoalkane
^both via nucleophilic substitution
Correct.
PCl5 (and for that matter, SOCl2 as well) doesn't react with phenol to generate aryl halides though, for 2 reasons.
1stly, the unlike aliphatic alcohols, the lone pair on the phenolic sp2 O atom is delocalized by resonance to form a pi bond with the sp2 C atom of the benzene ring in some resonance contributors), exactly as is the case with aryl and vinyl halides, and thus the resonance hybrid's phenolic O atom is electron-deficient (has a positive formal charge on some contributors, and hence a partial positive charge in the resonance hybrid), and thus is non-nucleophilic and cannot attack the electrophilic P atom of PCl5 (or the electrophilic S atom of SOCl2).
2ndly (even for the small % of phenols which happen to possess sufficient activation energy to attack PCl5 or SOCl2), the electron-rich Cl- nucleophile (eliminated from PCl5 or SOCl2 after reacting with ROH) is electrostatically repelled by the pi electrons of the electron-rich benzene ring nucleophile, and thus PCl5 and SOCl2 cannot complete the mechanism to convert phenol to aryl halides (which therefore does not happen).
If descriptions of the elementary steps are provided in the question, Cambridge could ask you (eg. in this year's A level exam) to draw the (partial or full) mechanism for PX5 or PX3 or SOX2 reacting with alcohols and carboxylic acids to generate alkyl halides and acyl halides respectively. So for those of you interested (and ready ; if any of these confuses you, then you're not ready and you should skip this entirely), here are the mechanisms
(The use of SOCl2 is often favoured over PCl5, because the SOCl2 reaction has an important advantage over the PCl5 reaction, can you guess what it is? Cambridge could ask this question sooner or later.
Ans : The SOCl2 reaction generates 2 gaseous products, while PC5 reaction only generates 1 gaseous product. Hence, the Gibbs free energy is expected to be more negative (ie. thermodynamically feasible) for the SOCl2 reaction compared to the PCl5 reaction, due to thermodynamically favourable positive entropy change.
Furthermore, since SO2 is a gaseous product, which readily leaves the solvated reaction mixture, hence the position of equilibrium is pulled over (or if you prefer, shifts more) to the RHS, as predicted by Le Chatelier's principle.)
Mechanism for SOCl2 and PBr3 reacting with alcohols to generate alkyl chloride and alkyl bromide :
https://www.khanacademy.org/science/organic-chemistry/alcohols-ethers-epoxides-sulfides/reactions-alcohols-tutorial/v/preparation-of-alkyl-halides-from-alcohols
Mechanism for SOCl2 reacting with carboxylic acids to generate acyl chloride :
https://www.khanacademy.org/science/organic-chemistry/carboxylic-acids-derivatives/reactions-carboxylic-jay/v/preparation-of-acyl-acid-chlorides
Mechanism for PCl5 reacting with carboxylic acids to generate acyl chloride :
https://upload.wikimedia.org/wikipedia/commons/7/71/Phosphorus_pentachloride_mechanism.png
Note that the phosphoryl chloride generated can be further hydrolyzed, this is covered in the H2 syllabus under the inorganic chemistry topic : periodicity. In fact, the mechanism for both water H2O (inorganic chemistry) and alcohol ROH (organic chemistry) reacting with PCl5, is exactly the same (up to a point), because did you notice that water (H-O-H) is a 'type' of alcohol (R-O-H) in which the R group is a H atom, and thus it should make natural and logical sense to you (did your school teach this to you?) that the reaction mechanism (up to a point) is exactly the same : HOH with PCl5 gives POCl3 and 2HCl, while ROH with PCl5 gives POCl3 and RCl + HCl.
You can try drawing the mechanism for further hydrolysis of POCl3 to give H3PO4 + 3HCl (notice that the OS of all elements remain unchanged during hydrolysis reactions, so based on the starting OS, you can deduce the final OS which will help you deduce the structure or formula of the final hydrolysis product). The mechanism involves 3 water molecule nucleophiles (one by one) attacking the electrophilic P atom of POCl3, thereby eliminating the 3 Cl- ion leaving groups one by one, followed by proton transfers to generate the final products of H3PO4 and 3 HCl. Cambridge could ask you to draw out this fairly simple mechanism (of which the balanced equation you have to memorize under the Periodicity topic anyway, so might as well you draw out the mechanism to make sense of it for yourself instead of blindly memorizing the equation like most JC students do... which is just sad. Learning should always be fun and meaningful.)
---------------------------------------------------------------------------------------------
Btw, the following is something extra, a bonus for those of you (eg. Flying Grenade) who may be interested in studying Chemistry at the Uni level. Did you know that other than SN1 (racemization) and SN2 (inversion of configuration), there's a 3rd mechanism called SNi (retention of configuration) whose secret existence, bewildered chemists figured out, when trying to figure out why some reactions like SOCl2 with alcohols mysteriously resulted in alkyl chlorides with retention of configuration instead of the expected racemization or inversion of configuration (ie. meaning it was neither SN1 nor SN2). If hearing this excites you, then yup, you should certainly consider studying Chemistry at Uni level. Enjoy! :)
SOCl2 and the SNi Mechanism
http://www.masterorganicchemistry.com/2014/02/10/socl2-and-the-sni-mechanism/ -
Originally posted by Flying grenade:
There is Shielding effect provided by electron filled orbitals (x/y/z orbital) experienced by the valence e- , albeit a smaller/weaker effect than a filled principal e- quantum shell is it?
Idk tat why i ask
Help!!!
"Is shieding effect provided from orbitals insignificant, only shielding effect provided by principal quantum electron shell significant?"
1st error : shielding effect is from electrons, not orbitals or shells.
2nd error : electron shells consist of orbitals, so you can't say shielding from shell more powerful than shielding from orbitals.
3rd error : there is no such thing as a "principal quantum shell". (Did your school teach you that? *sheads head*). There is only such a thing as "principal quantum number" and "electron shell". -
Originally posted by Flying grenade:
Cant find both cie and moe-cambridge mark schemes online thought they're restricted/confidential
U have? U are godly
Yes I am, but on a separate note, you can download the UK CIE papers from XtremePapers.com. The Singapore A level Mark Schemes are highly confidential and illegal to access. -
When describing trends of ionization energy and electronegativity across a period, Just keep it clear and simple and write, "Valence shell electrons are shielded from the positively charged nucleus most significantly from *inner shell* electrons; hence shielding effect remains approximately constant across a period." to get 1 mark when explaining the trend of ionization energy and electronegativity across a period.
The 2nd mark is stating "The number of protons in the nucleus increases from left to right across a Period, hence valence shell electrons are more strongly electrostatically attracted to the positively charged nucleus, and atomic radius decreases." and conclude with "Therefore, ionization energy becomes increasingly endothermic from left to right across a Period because of the increasing magnitude of energy required to remove an electron across a Period."
When asked to explain electronegativity trend across a period, the 2nd marking point is slightly modified to "The number of protons in the nucleus increases from left to right across a Period, hence valence shell *bonding* electrons are more strongly electrostatically attracted to the positively charged nucleus, and atomic radius decreases." and conclude with "Therefore, electronegativity increases from left to right across a Period because of the increasing effective nuclear charge.".
When describing and explaining ionization energy or electronegativity trends down a group, the common error taught by Singapore JCs, and for which Cambridge penalizes thousands of Singapore JCs students every year, is to say "effective nuclear charge decreases down a group". The moment you write that, you get zero marks for the question.
Instead, write "Ionization energy decreases down a Group despite increasing nuclear charge, for 2 reasons : due to the increasing atomic radius as a result of increasing number of electron shells, (not "principal quantum shells"!!!) hence as predicted by Coulomb's law, less energy is required to remove the increasingly further-from-nucleus valence shell electrons going down the Group; in addition, the valence shell electrons are also increasingly shielded from the positively charged nucleus by an increasing number of inner shell electrons going down the Group, thereby further reducing the magnitude of energy required to remove the valence shell electron to carry out ionization."
Of course, you don't have to phrase it exactly the way I did. Unlike Singapore JC teachers who anally insist you phrase your answer exactly the way they phrased it in their own lecture notes or else they'll penalize you in your Promo/MidYear/Prelim exams (and therefore you can expect JC teachers to also heavily penalize other JC teachers, both within a JC and across to other JCs, so few JC teachers will be able to get an A grade for Prelim papers when marked by other teachers from other JCs), Cambridge is *reasonable* (not lenient, but reasonable, there's a critical difference). So as long as you have the required Mark Scheme points, phrased in your own way (with some degree of creative freedom), you'll get the marks. -
Originally posted by Flying grenade:
Whats the diff bet side product and by product?
Go to my website by googling "BedokFunland JC", then look under my list of BedokFunland JC's A Level H2 Chemistry Questions. It's one of my questions, answers are provided therein. -
25 cm3 of NaHCO3 and NaOH titrated against 0.5M HCl. 21.5cm3 and 15cm3 of HCl required to reach 1st and 2nd equivalence points respectively. Find the molarities of NaHCO3 and NaOH.
Solution :
Molarity of NaOH = moles of HCl which neutralized NaOH / 25cm3 volume = (0.5(21.5/1000)) / (25/1000) = 4.3x10^-1 M
Molarity of NaHCO3 = moles of HCl which neutralized NaHCO3 / 25cm3 volume = (0.5(15/1000)) / (25/1000) = 3x10^-1 M -
Originally posted by Flying grenade:
Whats the difference between L (levo) and D (dextro) , And sinister and dexter isomers???? And also R and S isomers??
To answer your question, read :
https://en.wikipedia.org/wiki/Chirality_(chemistry)#Naming_conventions
For H2 Chemistry, if asked to name the enantiomers, Cambridge will accept either (+)/(-) or d/l labelling based on optical activity, or the R/S labelling based on the Cahn–Ingold–Prelog priority rules (which are also used for E/Z labelling system for alkenes, which is more versatile than the limited cis/trans system, and is also accepted by Cambridge for H2 Chemistry) though the R/S and E/Z labels are not compulsory for H2 Chemistry as it's not within syllabus, and only required for H3 Chemistry. The D/L labelling is used in biochemistry (H2/H3 Biology). -
Originally posted by gohby:
Hi UltimaOnline,
A Level 13/P2/Q5b(i)By convention when we write primary structure of the protein we do so from the N to the C terminus. Would it be wrong if I wrote the sequence from the C to the N terminus instead? Also, in this case, where is the C terminus?Thank you!
For oxytocin, there is no C terminus, because the C terminus (belonging to glycine) has reacted (in a nucleophilic acyl substitution, addition-elimination, condensation reaction) with NH3 to become the amide group you see at the bottom-left corner of oxytocin. You *must* write it the correct way around to get the 1 mark.
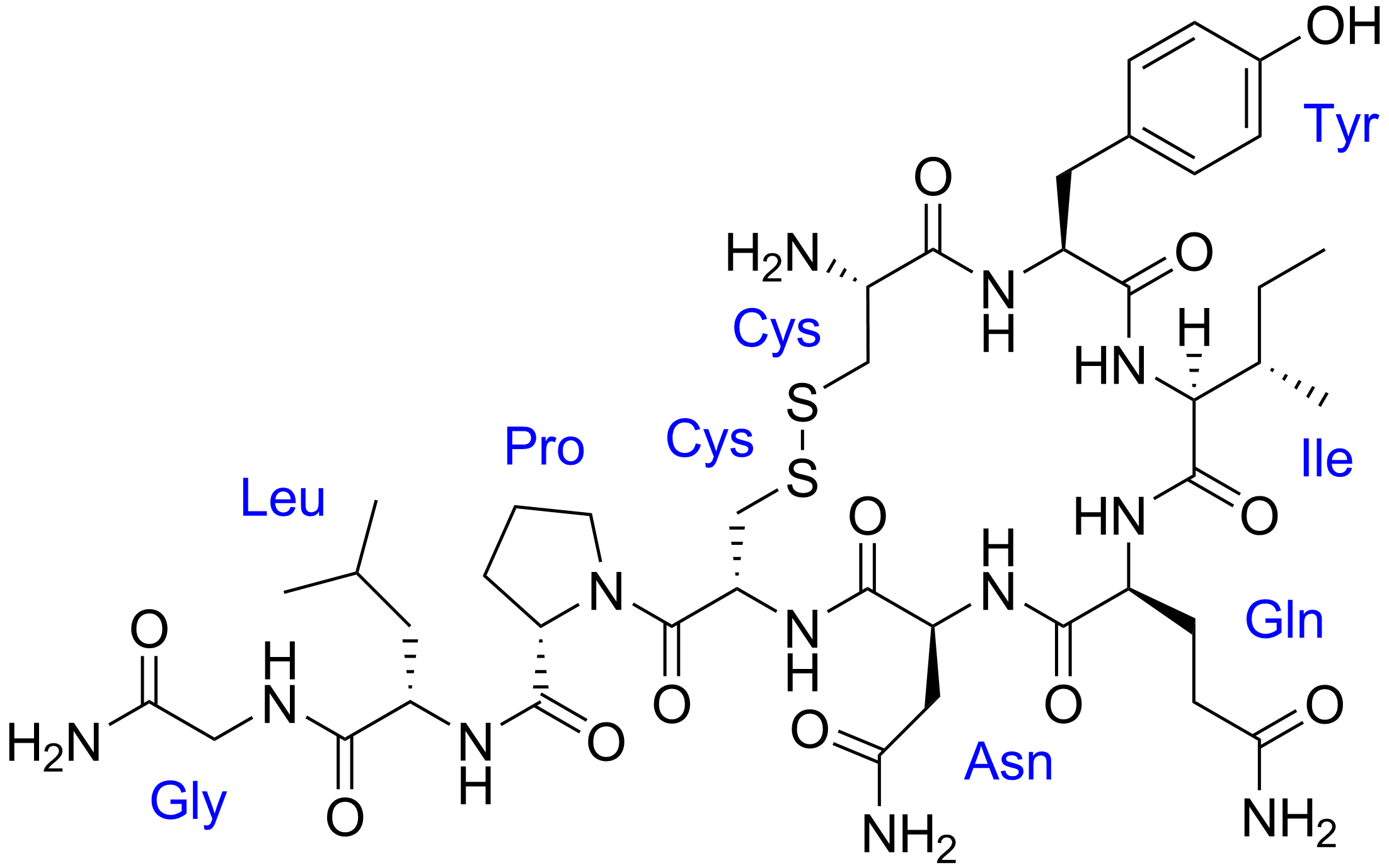
https://en.wikipedia.org/wiki/Oxytocin#Structure -
Originally posted by Flying grenade:
2010 p3 qn 1d
Sir ultima can help check my drawingsFor cpd A, the cl is substituted at that position is it because it is most stable there because attached to 3 mildly electron donating C group ah? Markovnikov’s rule ah?
For D, im asking this as an extension
When the Br is being eliminated, are there 2 other by-product as drawn?
Eh don't anyhow misuse his name. Markovnikov (Chinese for "horse cough you also cough") would turn in his grave if he heard you say that. Markovnikov's rule is only for hydrohalogenation via electrophilic addition across a double bond (if you know why it usually works to predict the major product, you'll also know when it'll fail, for challenging tricky qns). This is halogenation via free radical substitution, nothing to do with Markovnikov (he'll say 关我�事!). Because free radical substitution cannot be controlled, you'll get a mixture of many monohalogenated products. To determine which monohalogenated intermediate was used for this qn, observe the structures of the final product upon dehydrohalogenation (ie. this qn isn't asking you to state which is the major product, but rather identify the intermediate that could give you the final products drawn). But assuming you're jus curious if that's the major product of halogenation via free radical substitution, it *is* the major product, based solely upon the factor of stability of alkyl radical intermediate (in actuality, you need to mathematically combine both factors at a specified temperature to determine the actual major product). Then yes, your reasoning (though you wrongly labeled your explanation using Markovnikov's name) is correct : tertiary alkyl radical is more stable than secondary alkyl radical is more stable than primary alkyl radical. This factor is more pronounced for Br, less for Cl, and even less so for F (I is irrelevant here, because iodination via free radical mechanism cannot be carried out; you know why? asking you to explain why, was a Singapore TYS A level exam qn).
No, the dienes you drew cannot be generated. Why? Asking you to explain why, was 2014's Singapore TYS A level exam qn. Hints (any or all of these terms may be used in your explanation) : orbital hybridization, electron geometry, ring strain, angle strain, thermodynamic instability, thermodynamic infeasibility.
Originally posted by Flying grenade:Ohh!!
I only thought of that, due to the factor of ring strain.
Becos sp hybridised should have a angle of 180°, however in that case its bent at an angle.
Hence the diene cannot be formed
So yes, only 1,3diene can be formed right?
Can help improve and add in the rest of the factors into the answer pls
Thank u ultima!
You got the idea.
Cumulated dienes can indeed be generated, just not within a small ring like this molecule, because of the resultant ring strain due to angle strain (due to the significant deviation from the ideal bond angles about the sp hybridized C atom as predicted by VSEPR theory, which is to maximize thermodynamic stabilities by minimizing electron pair electrostatic repulsions) making it thermodynamically unstable and thus its formation thermodynamically unfeasible.
Conjugated dienes (such as the final product in this Cambridge qn) are thermodynamically favored due to the resonance stabilization energy (for the same reason why only benzene, but not the non-resonance mythical version, cyclohexa-1,3,5-triene, exists), which results in the resonance hybrid enjoying overall stronger C-C bonds (ie. 3 C-C bonds all with partial double bond characters, is overall still stronger and still has a more exothermic formation enthalpy compared to 2 full double bonds and 1 strictly single bond), a more exothermic formation enthalpy, and thus more thermodynamically stable and formation more thermodynamically favored.
Originally posted by Flying grenade:Which 2 factors?? Alkyl stability and temperature uh?
1st factor : No. of H atoms substitutable
2nd factor : Stability of alkyl radical intermediate, aka, reactivity of primary vs secondary vs tertiary H atoms.
Your own JC school teacher never teach you meh?
Go google up on how these factors influence product distribution during halogenation via free radical substitution, and the reactivity-selectivity principle (in the context of halogenation via free radical substitution, which requires an understanding of the Hammond postulate). -
Originally posted by Flying grenade:
Another godly answer.., thank you so much..!!!
Iodine is such a big molecule so it's unreactive?
The equation c2h5• +I• -> c2h5I +HI , is endothermic is it?
Oi, just saying "Iodine is a big molecule so it's unreactive" is a sure way of making the Cambridge examiner shake his/her head, "Singapore students only know how to smoke", don't xia suay Singapore hor.
Yes, correct, you must link to the fact that the bonds formed are weaker than the bonds broken (quote Data Booklet values whenever relevant), and hence the reaction is thermodynamically unfeasible.
Originally posted by Flying grenade:Isnt no. Of H atoms available for substitution same as tertiary, secondary or pri alkyl radical intermediate?
Is temperature a factor for FRS?
Not the same. In fact, the two factors are opposing. Take propane for example. Based on each factor, what is the expected major product? 1 factor predicts 1-halopropane as the major product, while the other factor predicts 2-halopropane as the major product.
If the Cambridge A level exam question requires you to calculate out the overall product distribution mathematically (which has appeared in past CIE A levels and Pre-U papers), the question has to provide you with the relative reactivities of a pri vs sec vs tert H atom (corresponding to the relative stabilities of a pri vs sec vs tert alkyl radical) in numerical values, for you to apply mathematically (ie. multiply both factors together). Such values differ across halogens, and across temperatures.
If your school JC teacher didn't teach you about these 2 factors (see the benefits of a Chemistry education @ BedokFunland JC ?), you can google up yourself, and/or read the following webpages :
http://orgchem.chem.uconn.edu/2443s2011/2443-041811.pdf
http://www.masterorganicchemistry.com/2013/10/31/selectivity-in-free-radical-reactions-bromine-vs-chlorine/
http://www.mhhe.com/physsci/chemistry/carey/student/olc/ch04radical.html
-
Originally posted by Flying grenade:
pH lower than pka, compound protonates
pH greater than pka, compound deprotonates
Which chapter is this??
Acid base equilibria?
Does this have any link to equilibria Le chatelier's principle shift left shift right?
I just memorise ^ only!
Hope i can draw links to reinforce
Yes of course it's acid-base equilibria, and your school JC teacher *did* teach you this, didn't he/she? It's basic (no pun intended) acid-base equilibria. Of course any equilibrium can be linked to Le Chatelier's principle, and also to Organic Chemistry (eg. acid-base equilibria of amino acids). Everything can be linked, once you understand Chemistry deeply enough.
-------------------------------------------------------
Originally posted by Flying grenade:2010 p3 qn 2b
Need to draw all the protein structures to answer this qn?
Diagrams of structures are optional (as indicated in the question phrasing). Cambridge will award you the marks if the required points are present in your answer, whether in words, in balanced equations, in your diagrams, or all 3. Unless you're supremely confident you can include all the required points are present in words alone, or equations alone, or diagrams alone, it's usually a good idea (assuming your time management is good enough) in such questions to use at least 2 out of the 3 forms in your answers (even if the question doesn't compulsorily require it), to ensure all the required points are present in your answer to secure full marks. So to address your question directly, no need, as long as the required marking points are present (go see a TYS publisher's answer for this). -
Originally posted by gohby:
I have a couple of O Level Science (Chemistry) MCQs on hand - would greatly appreciate if anyone could shed some light on these questions - cheers! :)
Answer: A
Remarks: Why is the answer A? I thought all of the statements are correct.
Remarks: The answer is B. However, how do I know that the [Fe3+]>[Fe2+], given that the question did not say that the precipitates were left to stand, thereby allowing for oxidation of Fe2+ to take place?
Answer: B
My postulation: The change in water level would mean that the gaseous pressure has increased. The answer can’t be D because ammonium chloride would be formed (so presumably the gaseous pressure exerted on the LHS would decrease since an ionic salt is formed). However, what will happen when CO or N is pumped into the set up? Am I right in saying that the answer is B because methane does not react with ammonia?
Q1. Diffusion also occurs in the solid and aqueous states.
Q2. Fe(OH)3 has a higher molar mass than Fe(OH)2.
Q3. Only CH4 has a lower molar mass than NH3. At constant temperature, the rate at which gas particles diffuse is inversely proportional to their molar mass.
-------------------------------------------------------------------------------------------
Originally posted by gohby:Hi UltimaOnline,
Thank you for your help :)
For 1 - how does diffusion take place in solids when the atoms are arranged in fixed positions? As for 3 I agree with what you mentioned, but how does it relate to the question?
Hi Gohby, no prob, as always :)
Q3. CH4 (being lighter) diffuses into the porous container faster than NH3 (being heavier) can diffuse out, resulting in an increase in gaseous pressure within the porous container.
Q1. This isn't taught at either O levels or A levels, but Truth is Truth, Chemistry is Chemistry, and diffusion *does* occur in solids (the thermodynamic motivation for diffusion, is of course, thermodynamically favorable positive entropy change, which is also the thermodynamic motivation for Le Chatelier's principle).
https://en.wikipedia.org/wiki/Mass_diffusivity#Solids
https://en.wikipedia.org/wiki/Atomic_diffusion
https://en.wikipedia.org/wiki/Effective_diffusion_coefficient
https://en.wikipedia.org/wiki/Lattice_diffusion_coefficient
https://en.wikipedia.org/wiki/Kirkendall_effect -
Originally posted by gohby:
Hi UltimaOnline,
I would like to seek your opinion/concurrence for some of the questions where my answers diverged from the answers in the answer scheme :) :Remarks: The answer is B but I got A. Given the number of moles of ammonium nitrate used is 0.1, the number of moles of ammonia produced should be 0.1 too, so I think the volume produced should be 2.4dm³. Btw, what is the role of the aluminium powder in the reaction?
Remarks: The answer is A. What should I be looking out for?
Remarks: The answer is A but I think the answer is B. As greater number of moles of lead (II) nitrate was present in Experiment II , there should be more lead (II) iodide formed in experiment 2, thus the total mass of lead (II) iodide is smaller in I.
Remarks:The answer is B but I think the answer is D. I would think this is an electrophilic addition reaction of an alkene rather than an FRS reaction.
Hi Gohby,
Your own answer for the last question about electrophilic addition of hydrogen halide across an alkene double bond is correct. Don't put too much trust in Singapore secondary schools' teachers' answers, or Singapore assessment practice books' answers, or Singapore TYS publishers' answers.
Q1. Al is oxidized to Al3+, and NO3- (OS of N = +5) is reduced to NH4+ (OS of N = -3). Thus a total of 0.1 + 0.1 = 0.2 mol of NH4+ is deprotonated by the OH- to generate 0.2 mol of NH3, which works out to be 4.8 dm3.
Q2. You need to use insoluble bases (ie. hydroxides and carbonates), as soluble alkalis are corrosive and will damage your mouth, throat and esophagus before it can reach your stomach to reduce acidity. (Medical note : aluminium is toxic and causes brain damage, thus shouldn't be used in drugs or additives, yet its use continues to be widespread, contributing to Alzheimer's disease and other related dementia medical conditions)
Q3. Either A or B could be correct, depending on the molarity of KI. If the molarity of KI is sufficiently low, it's *possible* for the mass of ppt generated in experiment 2 to be *less* than in experiment 1 (despite the moles of Pb2+ in experiment 2 being more than in experiment 1), because the total volume of solution in experiment 2 is greater. The secondary school teacher who set this question was being a smart-ass and must have been proudly confident of having set the trickiest O level Chemistry prelim paper question among all the secondary schools for that year (there's always an informal competition to set the toughest prelim papers, just look at the top JCs' prelim papers, most JC teachers themselves wouldn't be able to get an A grade if they sat for the top JCs' prelim papers within the allocated time). Unfortunately, he/she didn't realize that since the molarity of KI wasn't specified, if the molarity was sufficiently high, then even with a larger volume of solution, 99.9% of the moles PbI2(s) could be precipitated out in both experiments, then *your* answer would be correct. So this question has insufficient data to conclusively rule out either A or B. -
Originally posted by Flying grenade:
2010 p3 qn 4 gii
Photo is here
https://www.dropbox.com/s/morxcgb8519dx60/20151105_145329.jpg?dl=0
How can secondary alcohol oxidise to Carboxylic acid??
Thought secondary alcohol can only oxidise to ketone at maximum?
There are only 3 ways for a ketone to be further oxidized :
If it's part of a benzene ring side chain (with a benzylic H atom).
If you use a chemical oxidizing agent stronger than KMnO4, eg. concentrated HNO3.
If you burn it.