BedokFunland JC's A Level H2 Chemistry Qns (Part 2)
-
Originally posted by gohby:
Hi UltimaOnline,
Q1: "Fe3+ is more stable in acidic than in alkaline medium."
Remarks: How to I assess the veracity of this statement?
Q2: Referring to Q6 of posted on 15 Oct 2:51PM,
Which of the following gives the best description of the reactions of Group II metals and their compounds?
"Beryllium hydroxide is amphoteric due to the high charge density of the Be²+ ion."
I asked what is wrong with the statement as the answer. You mentioned "Q6. Replace the word "hydroxide" with "oxide", and you're good to go. The hydroxide is still significantly more basic than acidic."
I don't quite get it - both beryllium oxide and beryllium hydroxide are amphoteric - so what wrong with that description? Is it because it is not the best description?
https://en.wikipedia.org/wiki/Beryllium_oxide
https://en.wikipedia.org/wiki/Beryllium_hydroxide
Thank you! :)
Look at the Data Booklet reduction potentials. Is it easier (ie. reduction potential more positive) to reduce Fe3+(aq) or Fe(OH)3(s)? Thusly, determine the veracity of your abovementioned statement.
Fair enough, and my bad. Let me rephrase it this way :
For the oxide and the hydroxide of any metal to behave as a Bronsted-Lowry base, is obvious, as both the oxide and hydroxide anions have a propensity to accept protons to lower their anionic charge densities to stabilize themselves. Both the oxide and the hydroxide of Be are equally acidic, because the high cationic charge density of Be enables both the oxide and hydroxide to accept 2 *additional* moles of OH-(aq) (the oxide will first undergo hydrolysis to generate 2 OH-(aq), which are already present in the hydroxide ; subsequently for every mole of Be2+, 4 moles of OH-(aq) ligands complex with the Be2+ Lewis acid to generate the ionic tetrahydroxoberyllate(II) coordination complex), thus behaving as a (Lewis, not Bronsted-Lowry) acid.
The reason why I earlier suggested replacing the word "hydroxide" with "oxide" in your statement about amphotericity, is simply because the A Level H2 Chemistry syllabus focuses on the acid-base-amphoteric nature of the oxides, rather than hydroxides. So yes, you're right that both the oxide and the hydroxide of Be are equally amphoteric, but for A levels, it's the oxide that's tested rather than hydroxide, so if it were a P2 or P3 qn, I would still recommend you to write "oxide" rather than "hydroxide". Since we're left with P1, yes indeed, you (all students taking the A levels) should be aware that both the oxide and the hydroxide of Be are equally amphoteric. -
Ladies & Gentlemen, the 'A' grade boundary for 2015 A Levels H2 Chemistry has just shot up to 75%.
2015 H2 Chemistry Paper 1 MCQ Answers by BedokFunland JC1. B
2. D (for hydrocarbons, if "p orbitals" refer strictly to unhybridized p orbitals only) or B (generally, and for hydrocarbons too if "p orbitals" includes hybridized orbitals)
3. C
4. C
5. B
6. D
7. B
8. C
9. A
10. B
11. D
12. C
13. D
14. B
15. D
16. D
17. A
18. C
19. B
20. A
21. B
22. D
23. B
24. A
25. D
26. D
27. D
28. C
29. B
30. A
31. D
32. A
33. C
34. A
35. B
36. A
37. C
38. C
39. A
40. B -
Originally posted by studentxyz:
hi, just curious, but how do you estimate that you need 75% to get an A? because i keep hearing people say that you need more than 80% :(
Ok here's the thing : the way most students count their marks, assuming Cambridge will accept all their answers (eg. just because their school teachers or private tutors taught them to write that way), then you should play safe and go ahead and consider 80% to be the A grade boundary. But if you count your marks strictly (and err on the side of caution, ie. assume you didn't get that particular mark if you're not sure it's 100% acceptable by Cambridge), then following the Cambridge Mark Scheme (which I know) and the process of Cambridge computation of grade boundaries based on the bell-curve (which I know), then overall score > 75% is safe. I can't reveal any more details than this. -
Wynrox posted : "Unfortunately for me you are right for qn40... 😔 For qn 31 I was taught by my school that any ion with configuration 4s0 3d6 will become 4s1 3d5. Not sure which is correct though."
Yes totally unfortunately for you, but it's not surprising at all that I'm right.
That's true only for atoms in their ground states, not ions.
You can be sure I'm correct, though.
Fe2+ is [Ar] 4s0 3d6
Trust BedokFunland JC > Singapore JCs, ftw. -
Chloropicrin (IUPAC : trichloro(nitro)methane) was used in World War I as “vomiting gas� because it could penetrate gas masks and force soldiers to pull them off to throw up and expose themselves to other toxic chemicals.
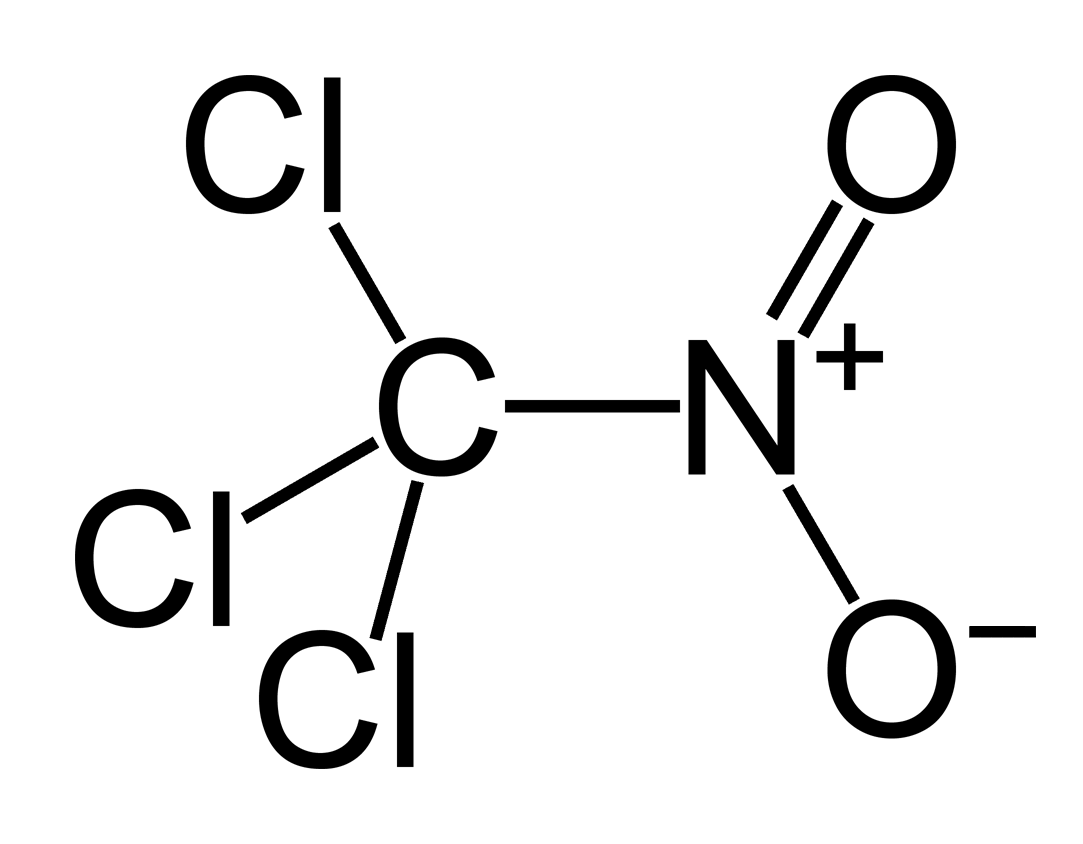
A level H2 Chemistry Qn : Suggest a synthesis pathway for Chloropicrin, and draw the mechanism for this pathway. -
Originally posted by hoay:
For a reaction whose rate= k [CH3CHO][CH3OH][H+]
exp [CH3CHO] [CH3OH] [H+] relative rate
1 0.20 0.10 0.05 1.00
2 0.25 0.10 0.05 1.25
3 0.25 0.16 0.05 2.00
Calculate the relative rate of reaction for a mixture in which the starting concentrations of all three reactants are 0.20moldm–3 .
Do we hve to use the rate expression for this ?
Since all 3 reactants are 1st order (as per the rate eqn), hence based on exp 1, the new rate of reaction will be : 1.00 x (0.20/0.20)^1 x (0.20/0.10)^1 x (0.20/0.05)^1 = 8.00 -
Originally posted by Shafiq_6480:
Hi UltimaOnline. I heard that there is a new syllabus for H2 Chemistry. How is it like? What are some of the changes?
Hardly any real changes for H2 Chem as far as the student is concerned (for MOE teachers, abandoning SPA is the real big change). Mostly just empty bureaucratic re-packaging of topics into "Core Ideas and Extension Topics", re-presentation of the "CURRICULUM FRAMEWORK", ie. how the topics are linked together thematically (something the H2 Bio syllabus already had all these years), plus some extra pedagogical bells & whistles (ie. more work for MOE teachers) called "Learning Experiences" and "Practices of Science" to (ostensibly) enhance the student learning experience and a deeper appreciation of the attitudes and ethical issues in the scientific approach. All of which are nothing to worry about (as far as the A level student is concerned).
Format wise, P1 now only has 30 MCQ instead of the existing 40 MCQ. P3 now has a Section B in which you choose 1 out of 2 qns (all Section A qns are compulsory), instead of the existing choose 4 out of 5 qns format. Planning is still 5% of the overall score, but has been shifted from P2 to P4 Practical Exam.
Which brings us to the biggest single change for the H2 Chem syllabus : SPA is being replaced by a one-time Paper 4 Practical Exam, due to ongoing problems with SPA since day 1 (which like so many ideas, sound good on paper but suffer from problems in practice), which I clearly foresaw when it was first implemented over a decade ago.
The syllabus content itself for H2 Chem is almost exactly the same, other than just adding and applying the thermodynamics Gibbs free energy formulae delta G = - n F Ecell (where F = Farady's constant 96485 coulombs) and delta G = - R T ln Kc (where R = Ideal gas constant 8.31446 m^3 Pa / K ), introducing the qualitative effect of the Nernst equation in electrochemistry, adding the Friedel-Crafts alkylation and acylation reaction conditions and mechanisms, adding the ortho-para vs meta directing nature of benzene ring substituents (ie. identifying electron-donating/withdrawing by induction vs resonance, and how these stabilize or destabilize the resonance hybrids of the different positional isomers of the tetrahedral sp3 intermediate of benzene during electrophilic aromatic substitution), plus a couple more little additions in some other topics.
None of which I haven't *already* been teaching my BedokFunland JC students all these years (and much more, so as to enable my students to more correctly understand, not just memorize, Chemistry, ie. giving my students the H3 Chem and Olympiad Chem advantage over H2 Chem students).
Update on 15 Jan 2016 : Oh yeah, 1 more little change in the new H2 Chem syllabus, Singapore A level students are now required to follow the new IUPAC Group numbers notation for the Periodic Table, instead of the A level simplified version of the CAS notation or old IUPAC notation. Eg. Halogens are now Group 17, not group VII. It's just a tiny insignificant change (nonetheless long overdue if you ask me), no worries. -
Racoon : "Candy floss for me? Wow thank you so much! Just lemme wash it first..."
BedokFunland JC : "O hydrogen bonding, thou art heartless... O positive entropy change, thou art cruel..."
Raccoon watching his candy floss disappear Vine is a metaphor for life's disappointments.
A helpless raccoon watches his hopes and dreams disappear before his very eyes and the internet can’t get enough of it.
Life can be full of disappointments but nothing sums it up quite like this clip of a devastated raccoon watching his candy floss treat suddenly dissolve away into nothingness in an instant.
Vine Video : http://www.telegraph.co.uk/news/newstopics/howaboutthat/12082869/Watch-Raccoon-candy-floss-Vine-video-is-a-metaphor-for-life.html -
Originally posted by gohby:
Hi UltimaOnline,
For this question, I am more interested in the mechanics of the experiment rather than the answer.
If I had understood this procedure correctly, it is the part where “saturating the solution with hydrogen sulphide� that is providing the S2- ions which is used to precipitate ZnS, followed by MnS.
A: Now, what is the purpose of passing HCl into the solution prior to the saturation of H2S? My thinking is that it suppresses the dissociation of H2S (by LCP), thereby ensuring that only a very minute of S2- is present in the solution when it is added to prevent both precipitates from forming at the same time, given their very small Ksp values?
B: Next, how is the pH raised during the experiment to allow for the maximum effective precipitation (just right before MnS starts to precipitate)? My understanding for such reactions is that OH- ions will be supplied to decrease [H+] but this is not stated in the question. Is my understanding correct?
C: As I add more H2S into the solution, does the pH increase? I think it does because, the dissociation of H+ ions from H2S is too insignificant to counteract the decrease of [H+] from the dilution, hence resulting to a lower [H+] and thereby a higher pH.
Yo Gohby!
A : Correct.
B : Correct.
C : Depends on the molarity of the H2S(aq) added. If H2S(g) is added, then the pH will still decrease (unless the solution is already saturated with H2S, then any additional H2S added to the solution will be forced to leave as H2S(g), so the pH won't change).
Originally posted by gohby:Happy New Year, UltimaOnline! :)
Why does the pH decrease with the addition of H2S? Wouldn’t the mixing of a strong acid (HCl) with a weak acid (H2S) result to a lower [H+], thereby increasing the pH of the solution?
Now if the pH does decrease with the addition of H2S, and given that we need to increase the pH of the solution to point when MnS starts to precipitate by adding OH-, procedurally how does the experiment work – I assume that I add the H2S and OH- simultaneously? The question doesn’t offer a very clear picture tbh.
(P.S: I am deviating so much from the original question and I feel that I am being too scholastic… but I think a sound understanding of how this experiment works will aid one in arriving at the solution..)
You too, Gohby! :)
For such experiments, you can saturate the soln first with H2S, then add OH- from eg. NaOH(aq) to control the pH to control precipitation. For this qn however, instead of adding OH-, the qn says you simply add H2S until sufficient S2- ions are present to precipitate out the less soluble metal sulfide. In other words, no OH- is added. You simply slowly add H2S until you get a ppt.
H2S is only acidic, thus even if it's a weak acidic, and a strong acid is already present, there's no way the pH can increase, unless the molarity is sufficiently low such that (as you previously mentioned) the dilution exceeds the proton dissociation.
Only when adding a basic (eg. S2-) or amphiprotic species (eg. HS-) to a strong acid, would the pH increase (as H+ is removed from soln by the amphiprotic species). But adding H2S (which is only acidic, not amphiprotic or basic) only serves to add more H+ into soln, thus decreasing pH further, but as stated previously, depending on (g) vs (aq), and depending on molarity of (aq) added. -
Originally posted by gohby:
Hi UltimaOnline,
In a mixture of 0.1M of ethanoic acid (pKa 4.8 ) and 0.2M of bromic acid (pKa 8.7), the initial pH is 2.90. Why is it that the initial pH of the mixture is solely based on the [H+] from the ethanoic acid, and not from the bromic acid? Wouldn't the different [H+] from the bromic acid affect the overall [H+] in the mixture? (The question does not state the proportions of the acids in the mixture.)
Next, the 2 acids are titrated with NaOH. In drawing the titration curve, I understand that the base will always react with the stronger acid first, then the weaker acid after the neutralisation with the stronger acid. However, what is stopping the base from reacting with the weaker acid at the same time when it was reacting with the stronger acid?
Thank you! :)
Yo Gohby,
First of all, the qn is erroneous. Bromic acid (Latin name) aka bromic(V) acid (Stock name) is a much stronger acid than ethanoic acid (Cambridge can ask H2 students to explain why). The qn was referring to hypobromous acid (Latin name) aka bromic(I) acid (Stock name), an acid weaker than ethanoic acid.
In which case, yes the pH of the soln is mostly controlled by the significantly stronger ethanoic acid. This is because as predicted by Le Chatelier's principle, the proton dissociation of the weaker acid is suppressed by the shifting of position of equilibrium by larger Ka of the stronger acid.
In other words, in excess H+ (from the stronger acid), the position of equilibrium shifts back to the undissociated HOBr. In this shifting of equilibrium position, Qa > Ka, (note that Ka value doesn't change because Ka is an equilibrium constant at constant temperature), hence position of equilibrium shifts from RHS, ie. BrO- + H+, to the LHS, ie. HOBr, until equilibrium is re-estabilished, ie. Qa = Ka.
But you're right that the weaker acid does still affect pH, and at Uni level Chemistry, may (depending on the magnitude of the difference in Ka values) still have to be taken into account for a more accurate calculation. For A level purposes, Cambridge will always choose acids that allow the A level approximation to be chemically and mathematically valid.
Nothing is stopping the base from reacting with the weaker acid at the same time when it was reacting with the stronger acid, but because this is an equilibrium, hence if the base deprotonates the weaker acid, equilibrium will take over to transfer another proton from the stronger acid to the weaker acid, concordantly and consequently in effect, it's as if the base deprotonated the stronger acid in the first place. Hence mathematically, we simply calculate based on deprotonating the stronger acid directly.
U're welcome ;) -
Originally posted by gohby:
Q1
As a follow up to the previous question, the question was correct as I omitted the (I) in the bromic (I) acid.
The answer indicates the precise volumes of sodium hydroxide required for during the 2 equivalence points as 10cm³ and 30cm³. However, wouldn’t that mean we have to assume that the mixture contains an equal volume of ethanoic acid and bromic (I) acid? Is this a reasonable assumption to make in light of the information provided?
Q2
For part (ii) my pH at equivalence point is 8.61 but the answer suggests 8.58. Did I overlook something? As for (iii), I am only able to get a pH of 11.946 (5sf).
I carried out my calculations using the exact values from the calculator. Alternatively, I would write 5sf for my intermediate workings and round it up to 3sf for the final answer. Both of them do not yield a pH of 12.0. If my calculations are correct, should I reduce the number of significant figures just to “confirm� the value of 12.0?
Q3(Ka of acid = 5.9x10^-4M)
I reckon this is a blind spot on my part, but using the formula Ka ≈ [H+]²/[acid] yields an answer of 0.169M instead of 0.179M as suggested by the answer. As for (ii) the answer suggests 0.0937M - I drew the ICE table but was unable to obtain 0.0937M exact - similar to the problem above. Is there something wrong with my calculation method and my degree of accuracy?Thank you once again, UltimaOnline :)
Q1. The question has to be interpreted to mean the stated molarities of 0.1M and 0.2M are not before mixing, but after mixing.
Q2. Question is erroneous (if taken as a new, unrelated qn to Q1) because the molarity of *either* NaOH or CH3COOH acid has to be given, but isn't.
(ii) If either molarity is given, then you can work out the moles, hence new molarity of CH3COO- ions, then applying Kb, you can work out [OH-] hence pOH hence pH.
(iii) An ICF (not ICE) table should be done to obtain the Final (the F in ICF) moles of OH- in excess. As predicted by Le Chatelier's principle, you can ignore the negligible hydrolysis of the much weaker base CH3COO-, and calculate pH based on the new moles, hence molarity, of excess OH- in solution.
Your values are close enough to the given answer. Cambridge (depending on question and required working) may accept a reasonable accuracy of 2 sig fig. The inaccuracy may lie with whoever set this qn, no worries. Just ensure your students have the correct underlying concepts.
Q3. Question is erroneous. Benzenesulfonic acid is considered a strong acid with a much larger Ka value than the question provides. To put that aside, let's assume we change the qn to a weak acid with the Ka you provided. Then both the answers you gave (ie. both your own and the stated answers) appears to be wrong. If you want to discuss this further, can you show detailed mathematical working on how you arrived at your answer? And what is the source of this qn? Which JC, which year?
A further problem arises : the assumption that has to be made at A levels (which Cambridge allows so as to not require a graphing calculator for H2 Chem) that equilibrium molarity can be approximated to initial molarity because change in molarity is much smaller than initial molarity for weak acid, no longer holds true for this question. Because the initial [H+] is too large as the initial pH is too acidic. As such, graphing calculator to solve quadratic equation is required, and hence this question will not be asked at A levels.
For (ii), from Ka of HA, calculate Kb of A-. Hence from Kb formula, plugging in the [OH-] (calculated from pH read from graph), you can work out the [A-], which solves the question. If you did this, ie. your approach is correct, then no worries about the mathematical discrepancy. It may likely be the question setter's fault, not yours. -
Originally posted by gohby:
Hi UltimaOnline,
Many thanks for your reply.
Q2 (YJC Prelims 04 P3): Urgh my bad. The Ka of ethanoic acid is given as 1.8x10^-5M. It was embedded in part (a) of the same question which was unrelated to part (b), save for this Ka value, so I extracted part (b) in order not to clutter my question, but I forgot to include the Ka value from embedded in part (a).
I think these are standard questions; normally I am not very concerned about slight deviations with the answers. However this question specifically required the confirmation that the pH is 12.0, and I could only get 11.946 (and 8.61 for part (ii)).
For Q3, this is a HCJC Prelims 02/P3 question. My workings are as follows:
Given that the initial pH of the acid is 2.0, so [H+] = 10^-2. I used the formula Ka ≈ [H+]²/[initial acid], i.e. 5.9x10^-4 = [10^-2]²/ [initial acid]. So I found [initial acid] to be 0.16949M (5sf).
My detailed workings for part (b) are as follows:
Kb = 10^-14/5.9x10^-4 ≈ [OH-]²/[sodium benzenesulfonate]
Since pH at equivalence point is 8.1, [OH-]² = 1.5849x10^-12. Hence [sodium benzenesulfonate] = 0.093509M. However the answer provided was 0.0937M.
Ah ok Gohby, no worries then, your working is fine. It's likely that the question setter used a slightly different (eg. 3sf instead of the given 2sf) value for Ka, in his/her own calculations. Another possibility could be related to my next point.
What I've pointed out earlier, still holds. Benzenesulfonic acid should have a much larger Ka (and Cambridge can give a few Ka values of other acids and ask the student to deduce, with reasons, which is the correct Ka of benzenesulfonic acid). And if the initial [H+] is so high, the approximation is no longer valid. In other words, you must use the formula Ka = ([H+] x [A-]) / (initial [HA] - [H+]).
Perhaps this is what the question setter did. You can try it out to see if the answer is closer to the question setter's answer (and explain to your students why this is a more correct working ; ie. because the proton dissociation is no longer negligible compared to the initial molarity of the acid, hence the approximation is no longer mathematically valid to 3 sf).
No prob! ;) -
Originally posted by gohby:
Hi UltimaOnline,
Your explanation was spot on! For 3(i) when I calculate the pH without the approximation I got the answer to be 0.179M. :)
However, how do we ascertain if the [H+] is sufficiently high for which the approximation would not be valid? Separately, when we calculate the pH at the equivalence point are there circumstances where we cannot approximate the [salt] as the intial concentration of the salt as the dissociation is deemed to be significant?
Nice :)
Actually there are 2 reasons why approximation should not be used for this question (as well as a similar Cambridge question asked over 10 years ago).
Firstly, as I said, [H+] in Change is not << initial [HA], hence equilibrium [HA] can't be approximated to initial [HA]. That pH is 2.0 (furthermore by the structure benzenesulfonic acid, you can expect it to be a strong acid) should sound a warning bell that [H+] won't be small enough for the approximation to be valid. Mathematically, initial [HA] should be at least 100x larger than [H+] dissociated for the approximation to be valid to 3 sig fig.
Secondly, the practical reason why Cambridge allows the approximation is because H2 Chem students are not expected to have graphing calculators with them. And nobody is expected to solve quadratic equations without a graphing calculator or app these days. And notice that for this question (as well as the Cambridge question over 10 years ago), there was no need to solve a quadratic equation (since you only get x, not x^2). Hence, there is no excuse (eg. "I don't have a graphing calculator!") to use an approximation in the first place, so full credit would only be given if the correct, non-approximation working was used.
Regarding salt hydrolysis, same thing. Treat it as any weak base. If the [A-] used up by hydrolysis (indicated by Kb value) is too large to approximate equilibrium molarity back to initial molarity, then graphing calculator has to be used to solve for quadratic. In this case, Kb value is so small, the [A-] used up hydrolysis will be much smaller than initial, hence approximation is valid, graphing calculator is not required.
Edited on 16 Feb 2016 to add the following related question by Flying Grenade :
Originally posted by Flying grenade:Hi ultima, one of your post(that i lost the link i couldn't find) you mentioned about that one cannot assume that initial [H+] approx the concentration at eqm, because initial [H+] is too great. And a quadratic equation arise. But we can indeed use graphic calculator or scientific calculator to solve quadratic equations as they are approved for use in exams
I didn't say you're not allowed to, I just said you didn't have to. In other words, for A levels, Cambridge will set the questions such that the initial molarity is >> than the change in molarity, such that the equilibrium molarity can always be approximated to initial molarity, since Cambridge won't force you to use graphing calculator for H2 Chem. (But if your school exam papers use Uni level Chem questions, as Singapore JCs often do, such that the initial molarity is not >> than the change in molarity, then for such questions, you're not allowed to approximate equilibrium molarity back to initial molarity, and you *must* use graphing calculator to solve the quadratic equation which arises).
There is one type of A level exam question which is the exception though, that still doesn't require you to use graphing calculator, but does require you *not* to approximate equilibrium molarity back to initial molarity. Such a question was raised by Gohby a few weeks ago, and another such question was asked before by Cambridge (for Singapore A levels) over a decade ago.
If the pH at equilibrium (eg. at initial, at equivalence point, etc) is given to you (either stated in the question or graphically), then change in molarity of H+ (or in some questions OH-) can be thus derived (ignore negligible contribution by the self-ionization of water), which means there's no need to solve any quadratic equation and hence no need for graphing calculator, in which to calculate the equilibrium molarity (or in Gohby's question, the initial molarity) of the weak acid (or in some questions weak base), you have to take the initial molarity, minus away the known (ie. non-algebraic) change in molarity to dissociate to generate H+ (or in some questions base hydrolysis to generate OH-), to get an accurate value for molarity of the weak acid (or in some questions weak base) at equilibrium (or in Gohby's question at initial), in order to (as was the case in the Cambridge Singapore A level question a decade ago) calculate Ka or pKa (or in some questions Kb or pKb) as required by the question. -
Originally posted by Flying grenade:
what are the reason behind the group numbers??
tried to research but to no availfrom bbc bitesize
https://www.dropbox.com/s/mtfhzok1d1jmh46/Capture.PNG?dl=0
: Elements in the same group in the periodic table ... their atoms have the same number of electrons in the highest occupied energy level.from bmat specimen paper
https://www.dropbox.com/s/0nihqt0kd17dzqt/Capture2.PNG?dl=0
: the number of the group shows the number of electrons in the outer shell of the atom;how are these true??
e.g. Neon :1s2 2s2 2p6 3s2, 3p6
number of e- in highest occupied energy level = 6 , number of e- in the outer shell of atom= 8 , 18
??
18
??
This is true specifically for d block elements, including transition metals. Eg. Cu is Group 11, since it has 4s1 3d10 electron configuration, ie. 1+10 = 11 valence electrons. For d block elements, the 3d subshell is considered part of the valence shell, since 3d electrons participate in bonding. -
Originally posted by CKTR:
Err, chemistry noob here, pls help. For chemistry, how do you preduct the product of the reactions if only given the reactants?
For example,
If Lead(II) sulfide is reacted with hydrogen peroxide , why is the product lead(II) sulfate + water but not lead (II) sulfate + hydrogen. (Adopted from CS TOH Alevel practise questions)
Can anyone please tell me the what are the steps of predicting the product of the reaction?
Unlike O levels, now that you're entering A levels which is preparation for Uni level, there is no simple shortcut way of predicting the product of a chemical reaction. You must study all the different topics and properties of different species, and apply what you've learnt across all topics into answering a question.
H2O2 is a strong oxidizing agent, and hence is able to oxidize the sulfide ion to the sulfate(VI) ion, and H2O2 is reduced to H2O in the process. Due to the inert pair effect, it won't be easy to oxidize Pb2+ to Pb4+ or PbO2. And S2- can be oxidized to S, S2O3 2-, S4O6 2-, SO3 2-, S2O8 2-, or SO4 2- (there exist other sulfur containing ions with other OSes, but unless the question includes such data, stick to the common ions you're expected to be familiar with). And since the reduction potential of H2O2 to H2O is positive and of a sufficiently large magnitude, the cell potential for the combined redox reaction will be positive and hence thermodynamically favourable for the oxidation of S2- all the way to the maximum OS of +6 for sulfur (and SO4 2- is more stable and is hence the logical, most probable product, compared to S2O8 2- due to the peroxy / peroxo group with OS of -1 for oxygen, which you can deduce won't be present in the final product because the reduction of the peroxo group in the H2O2 reactant is the thermodynamic driving force behind the entire redox reaction in the first place).
As to your qn why you don't get H2(g), at A levels you have to be aware that to reduce H or H+ from H2O2 or H2O into H2 gas, you'll first need to either obtain a significantly large quantity of H+ (but neither H2O2 and H2O are sufficiently acidic to be readily deprotonated), or you'll need a stronger reducing agent than S2-, and no other reducible species that will take priority over such (in this case the peroxo group takes priority for reduction). Also, bear in mind you need a highly reactive (ie. electropositive) metal to reduce H+ (from H2O or H2O2) to H2(g), but in your CS Toh question, the reactant is Pb2+, not Pb(s). Pb2+ is already in a stable oxidized state, and hence is unable to reduce H2O or H2O2 into H2(g). And even if it was Pb(s), the oxidation potential of Pb to Pb2+ is not sufficiently positive to reduce H2O or H2O2 into H2(g), unless acidic H+(aq) is already present. Furthermore lead readily reacts with oxygen and/or water vapour in the atmosphere, to form a passivation layer of insoluble PbO(s), posing a further barrier to the reaction with H2O or H2O2. Strong acids are required to first protonate and dissolve away the PbO(s) passivation layer, to react with the Pb(s) underneath, and only then, would you be able to obtain some (not vigorous effervescence though) H2(g). -
Originally posted by CKTR:
Will all these stuff be taught at later stages or am I expected to know all these as H2 chem prerequisite? Btw thanks for your explaination.
Ps Qns is adopted from CS TOH qns 8 (stoichiometry, moles section)
For the A level exams, most will be taught as part of the basic H2 syllabus, some you'll be expected to apply existing knowledge to figure out, and a couple will be slightly beyond the basic H2 syllabus that are intended only for the distinction A grade students to be able to answer (ie. the A grade qns to 'distinguish' A graders from the rest of the cohort).
In your particular case, the question would have included sufficient data (eg. descriptions of the products for you to deduce their identities) for you to write and balance the equation to apply stoichiometry. Because such a qn (in CS Toh's practice book under the topic of stoichiometry) would primarily focus on testing your stoichiometry skills, as you would not yet have learnt the rest of the syllabus to be able to figure out all the products by yourself. -
Originally posted by nicolemantou:
ClCH2COCL + NH2CH2CH2OH -> E -> add Na and warm -> F
What is E and F?
First identify which is the stronger nucleophilic group (amine vs alcohol) and which is the more reactive electrophilic group (alkyl halide vs acyl halide). Cambridge may ask you to explain their relative reactivities.
Hence, nucleophilic acyl substitution first occurs to generate amide (instead of ester). Next, Na deprotonates OH, the subsequent alkoxide conjugate base nucleophilically attacks the alkyl halide via SN2 (both intramolecularly and intermolecularly are possible, especially when such a large ring involves less ring strain due to angle strain).
If the question doesn't provide additional data or conditions in regards to the product, the exam-smart student will give both answers in the A level exams, with labels as to which is the intramolecular SN2 product and which is the intermolecular SN2 product. -
Originally posted by nicolemantou:
Why is the product of SN2 inverted from that of the starting organic molecule?
Animation for SN2 inversion of configuration :
https://www.youtube.com/watch?v=o7hnYlfwMMM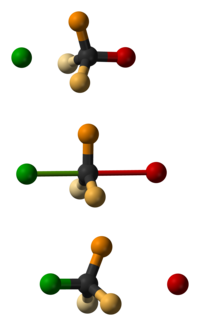
Source : https://en.wikipedia.org/wiki/Walden_inversion -
Originally posted by nicolemantou:
Why is CH3CH2CH(OH)2 unstable?
Because it is a geminal diol, whose close proximity of the 2 OH groups allow elimination of H2O to occur with thermodynamically favourable positive entropy change.
-
Originally posted by Ng.keebin:
Compare and contrast the reactions of ethanol and phenol with each of the following reagents:
a) ethanoic acid
b) potassium manganate (VII)
Phenol is too weak a nucleophile (due to resonance delocalization of the lone pair) to attack the weak electrophile ethanoic acid, hence esterification does not occur, as it would with ethanol.
KMnO4 is able to oxidize ethanol, but not phenol, due to the absence of an alpha H atom that has to be eliminated during oxidation (when you eliminate a less electronegative atom, the bonded atom's oxidation state is forced to become more positive).
-
Originally posted by Ephemeral:
Why do we look at strength of conjugate base when determining acid strength?
To be precise, it's recommended (for A level exams) that you analyze the *stability* of the conjugate base, rather than the strength of the conjugate base. Of course, they're related insofar as the more stable the conjugate base (or any base), the weaker it is as a base.If after buying that million dollar Ferrari, you don't have any money left to eat, obviously you wouldn't buy it in the first place. If after buying that million dollar Ferrari, you still have lots of money left, then naturally you wouldn't mind buying the Ferrari in the first place, coz you can afford to buy it.
If after donating a H+, the conjugate base is highly unstable, then it wouldn't be willing to donate the H+ in the first place, and therefore the more unstable the conjugate base, the weaker the conjugate acid.
-
Originally posted by Loh.huiyuen:
What are alcohols? What are the drinkable alcohols? Why are some alcohols not drinkable?
Only ethanol is drinkable (and even ethanol is considered slightly toxic). All other alcohols are a lot more toxic to humans, with the most common culprits of alcohol poisoning (due to the fact that they are readily available in non-consumable household or industrial products and subsequently used unethically and illegally as cheap and readily available replacements for ethanol to sell as drinkable alcohol to unsuspecting victims) being methanol and propan-2-ol. Specific technical details on why the different alcohols are toxic and their biochemical mechanisms in the human body require a biochemical and medical background to understand.
https://en.wikipedia.org/wiki/Alcohol#Toxicity
https://en.wikipedia.org/wiki/Methanol#Toxicity
https://en.wikipedia.org/wiki/Isopropyl_alcohol#Toxicology -
Originally posted by Liying98:
https://twitter.com/itsliyingg/status/693776550546673664
phenol ; aldehyde or ketone ; COOH group since C contains 4 O atoms (and 2 O atoms are accounted for by aldehyde/ketone and phenol) ; based on the difference in molar masses between C and product of rxn 2, you can deduce that 2 H atoms have been substituted by 2 Br atoms ; PCl5 first converts COOH group to COCl electrophilic group, which is subsequently attacked by the nucleophilic phenolic group in an intramolecular nucleophilic acyl substitution (mechanism is addition-elimination) to generate the cyclic ring, and you know the ketone group must be between the benzene ring and the COOH group, so as to prevent excessive ring strain due to angle strain and allow the cyclic ring to form in the condensation, addition-elimination, nucleophilic acyl substitution reaction. -
Originally posted by Ng.keebin:
Salicyclic acid contains both a hydroxyl and a carboxylic acid group. Why will the hydroxyl group bonded directly to the benzene ring not be substituted upon addition of SOCl2?
 Image : https://en.wikipedia.org/wiki/Salicylic_acid
Image : https://en.wikipedia.org/wiki/Salicylic_acid
Simple answer :
Because only aliphatic alcohols (and carboxylic acids) react with SOCl2 and PCl5, but not phenols.
Further answer :
Due to the sideways overlap of p-orbital of the O atom and the pi-orbital of the benzene ring, a lone pair on the O atom is delocalized by resonance to form a pi bond with the sp2 C atom of the benzene ring, giving the C-O bond in the resonance hybrid partial double bond character, which is thus strong and does not cleave readily. Hence all such nucleophilic substitutions on the benzene ring (which involves the potential leaving group having partial double bond character with the benzene ring in the resonance hybrid) are strongly resisted.
Even deeper further answer :
In addition, depending on the solvent used (eg. nucleophilic or not) and any other reagents present (eg. pyridine in SOCl2 reaction), let us consider the following 6 nucleophilic substitution mechanisms for the nucleophilic substitution reaction between phenol and SOCl2 or PCl5 : SN1, SN2, SNi (nucleophilic substitution with internal return), and Nucleophilic Aromatic Substitutions via either SNAr (addition-elimination) or via the benzyne intermediate (elimination-addition) or via SRN1 (free radical nucleophilic aromatic substitution).
SN2 isn't possible for phenols because the electrophilic C atom is itself part of the benzene ring, which hence poses an insurmountable steric hindrance (ie. it is geometrically, sterically and electrostatically impossible for the electron-rich Cl- nucleophile to approach the electrophilic C atom from within the electron-rich benzene ring itself as required by the SN2 mechanism) and concordantly, generating a geometrically unstable and thermodynamically infeasible pentavalent transition state (bear in mind this is nucleophilic substitution, not nucleophilic addition).
SN1 and SNi for arenes are highly thermodynamically unfavorable because upon attempted elimination of the leaving group (ie. the addition product of the phenolic group with the SOCl2 or PCl5 reagent), the positive formal charge on the consequent (highly unstable and thus thermodynamically infeasible to form) aryl carbocation species is highly destabilizing for 2 reasons : the positive formal charge would be on a sp hybridized C atom (ie. high % of s orbital character results in increased electronegativity which exacerbates the destabilizing effect of a positive formal charge), and furthermore the positive formal charge can *not* be delocalized by resonance (which is only possible for benzylic carbocations and carbanions, ie. when the formal charge is on the C atom outside, not within, the benzene ring), as the ring strain due to angle strain (ie. destablization due to significant deviation from the ideal bond angles of the sp hybridized C atom as predicted by VSEPR theory) prevents formation of an allene resonance contributor.
Nucleophilic aromatic substitutions do not readily occur and have high activation energy barriers (do you know why? Cambridge may ask you to suggest reasons for this). SNAr (addition-elimination) mechanism requires (preferably 2 or 3) strongly electron-withdrawing by both induction and resonance groups (preferably NO2 nitro groups) at the ortho and para positions (in salicylic acid, the ortho COOH group is indeed electron-withdrawing by induction and resonance, but not as strongly as NO2 groups because a formal positive charge on the N atom is more strongly electron-withdrawing by induction compared to a mere partial positive charge on the C atom).
The benzyne intermediate (elimination-addition) mechanism requires a very strong base for deprotonation of benzene (usually employing the amide NH2- ion, in which case the final product would be phenylamine rather than chlorobenzene) and the benzyne intermediate is itself highly unstable due to reasons discussed earlier (ie. destabilizing ring strain caused by angle strain due to significant deviation from the ideal bond angles about a sp hybridized C atom as predicted by VSEPR theory resulting in an unusually weak triple bond and hence being thermodynamically unfavorable), with equally unstable cumulene and biradical resonance contributors.
SRN1 (free radical nucleophilic aromatic substitution) requires a free radical initiator, and for which halogens are the leaving group to be eliminated rather than the incoming nucleophile. -
Further reading for those of you who might be interested in the stereochemistry of the SOCl2 (thionyl chloride) mechanism :
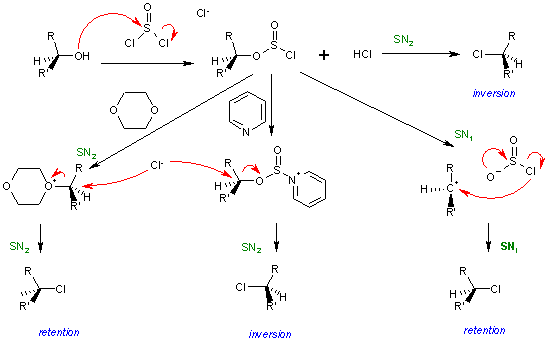
Image source : https://en.wikipedia.org/wiki/SNi
If you have difficulty understanding the diagram above, James Ashenhurst explains the reaction in detail here : http://www.masterorganicchemistry.com/2014/02/10/socl2-and-the-sni-mechanism/