BedokFunland JC's A Level H2 Chemistry Qns (Part 2)
-
Originally posted by Flying grenade:
Nitration of methyl benzene occurs more readily than Nitration of benzene , because ch3 group donates e- into the benzene ring, hence making it more nucleophilic ah?
Yes of course, but you didn't specify "electron donating" by which modality, induction versus resonance.In answering A level exam questions, you're advised to specify "electron donating by induction" or "electron donating by resonance" or "electron withdrawing by induction" or "electron withdrawing by resonance".
-
H2 Chemistry students are often confused between Iodometry and Iodimetry, the 2 commonly encountered types of redox titrations involving iodine. If you're uncertain of the difference, go check it up yourself now.
Tis the Age of the Internet,
Google by thy Sword,
Wikipedia be thy Shield- BedokFunland JC
-
Originally posted by Flying grenade:
What about secondary alkyl halide?
Teo Chee Hean : "What do you think?"
Tis the Age of the Internet,
Google by thy Sword,
Wikipedia be thy Shield- BedokFunland JC
-
Originally posted by Flying grenade:
Hi ultima, may i ask if i could enlist your help,
https://www.dropbox.com/s/jidtc1u05d4hbcp/20160331_194130.jpg?dl=0 , for this no link qn
Qn 1b)
Is this tutorial qn epic fail or is it a legitimate qn?
Im not sure how bronsted-lowry theory of acid and bases can apply to this qn, cant visualise any H+ donated or accepted
Thanks, Ultima
Update : is it H2O dissociates to form OH-and H+ , while NaH dissociates to form Na+ and H- , thereafter NaOH is formed from Na+ and OH- , while H2 is formed by H+ and H-
Since H- accepts the proton (H+) from NaH, is it that the proton donor i.e. bronsted-lowry acid is NaH?
Im also confused because i know amphiprotic water is both a weakacid and weak base,
So I'm deeply conflicted with the qn, not sure how do do this qn
Thanks for the help if possible, Ultima!
To properly understand this reaction, you *must* (so the sooner you accept this fact and work favorably with it, the better for your own sake) draw out the mechanism, which Cambridge can (and hopefully will, soon) ask H2 Chem students to do so, albeit as an A grade distinction challenging question to discriminate the elite Chem students from the rest of the cohort.Hydride ion, H-, from NaH (bear in mind that being ionic, it exists as Na+H- in the solid or solvated-in-inert-solvent state) is the Bronsted-Lowry base, which abstracts the proton H+, from the H2O molecule, which is the Bronsted-Lowry acid.
I expect all my BedokFunland JC students to be able to draw out the mechanism (usually only taught to H3 Chem and Olympiad Chem students, but if as a H2 Chem student, you can't draw out the mechanism, then you're not truly understanding this reaction, just blindly following 'rules' or 'patterns', such a tragedy for Singapore schools' H2 Chem students), otherwise (if you can't draw out simple mechanisms like these, then) you have no right to call yourself a BedokFunland JC student.
-
Originally posted by Flying grenade:
T_T why for WeakAcid , at eqm, [HA] approx [HA]initial ?
Is it because HA only partially dissociates, then can just assume conc approx concentration initial?
Is it always the case?
That's actually the case only for very weak acids or bases AND you start with a relatively large initial molarity of the weak acid or base, where hence the change in molarity is much smaller than initial molarity, and therefore the approximation is mathematically valid, otherwise you need to use graphing calculator to solve quadratic equation.For A level purposes, because Cambridge wants to leave the testing of mathematics to H2 Math instead of H2 Chem, and also because Cambridge is worried not all H2 Chem students are H2 Math students who can afford graphing calculators, therefore Cambridge has stated that they will always select acids or bases that are so weak, and/or start you off with a relatively large initial molarity of the weak acid or base, such that the approximation of equilibrium molarity back to initial molarity will always be mathematically valid, so no worries for A levels.
However, in some rare questions, eg. when the pH at equilibrium (eg. equivalence point) is given to you and you're tasked to solve for Ka or pKa or Kb or pKb, then because you need NOT solve quadratic equations, hence (by right) you have NO excuse to use approximation, then (by right) you will NOT be allowed to use approximation (again this is by right, but Cambridge tends to be lenient to H2 Chem students in this regard, and MAY accept approximation, but by right there's no valid reason to approximate, since there's no need to solve quadratic equation, so you're advised to give the correct answer without approximation).
Such questions have been asked in both Singapore JC Prelim paper questions, as well as by Cambridge many years ago, but not in recent years. Nonetheless, do be prepared.
-
Originally posted by Flying grenade:
Ultima, may i ask for your help,
https://www.dropbox.com/s/ic7dycmt7rj35h4/20160331_234915.jpg?dl=0
Qn 7 a iii)
I can only think of common ion effect(CIE), CIE causes the dissociation of a salt to be reduced, if the solution already contains one of the ions. which i think might be wrong?Cos CIE only affects salt is it? Can it apply for Weak acid/bases ?
I thought of another
Or is it because the [CN-] increase with the addition of NACN , then the by le chatelier's principle, eqm shift back to the left, causing formation of [CN-] at the R.H.S of equation to be less ah?
Help ):
A couple of things. Don't use acronyms in the A level exams. CIE more properly stands for "Cambridge International Exams" not "Common Ion Effect". Next, it is actually wrong to say "by Le Chatelier's principle" as commonly taught in Singapore JCs, the correct phrasing is "as predicted by Le Chatelier's principle", since Le Chatelier isn't some supernatural being who can magically shift the position of equilibrium in chemical reactions just by invoking his name.Also, and again despite what your school teacher may have said, Cambridge will never require, nor award marks, for the phrase "Le Chatelier's principle" in the A level exams. Otherwise, students who have no idea how to solve the question, other than knowing the obvious that it's an equilibria question, will just write the words "by Le Chatelier's principle" and hope to smoke out 1 mark out of nothing.
Common ion effects applies to all ionic equilibria, just as common species (including ions) effect applies to all equilibria reactions, as predicted by Le Chatelier's principle.
Correct Answer : When NaCN(s) is added to the solution, [CN-] increases, causing the position of equilibrium in the proton dissociation equation HCN(aq) (insert double half-arrow) H+(aq) + CN-(aq) to shift to the LHS. Concordantly, the extent or degree or percentage of HCN proton dissociation will decrease, and the pH of solution will become less acidic, compared to if no NaCN(s) was added.
-
Originally posted by Flying grenade:
Heard from my cher that the syllabus is changing the room temperature, no longer 25degrees C, changing to 27 or 28
Yes, due to global warming. And the fact that today is April Fool's day. -
A BedokFunland JC H2 Chemistry Qn
Which molecule (phenylamine or pyridine) is a stronger Bronsted-Lowry base? Explain your reasoning.
 versus
versus 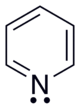
-
Originally posted by Ephemeral:
What is a simple chemical test to differentiate between 2,4,6-tribromophenol and 2,4,6-trichlorophenylamine? (Cannot use FeCl3)
BedokFunland JC to the rescue :Vary the pH, and observe (with your eyes) which precipitate dissolves at which pH.
-
All JC students intending to study Medicine should watch Dr House (8 seasons complete). Here's a snippet on "Why you should not bet with Dr House".
-
Originally posted by Flying grenade:
repulsion : lone pair-lone pair > lone pair-bond pair > bond pair-bond pair
where does lone pair-lone electron , and bond pair-lone electron repulsion fit in to the above inequality?
Bear in mind that for alkyl radicals (ie. unpaired electron on a C atom), the unpaired electron occupies an unhybridized p orbital, orthogonal to the trigonal planar sp2 hybridized orbitals, while the three sp2 hybridized orbitals are used for sigma bond formations with adjacent C or H atoms.For other cases, you may take it that an unpaired single electron occupies less space and has less repulsion than either a lone pair or a bond pair.
This explains the 134 degrees bond angle of NO2 (in which the unpaired electron occupies a sp2 orbital, but has less repulsion with the bond pairs, hence bond angle increases slightly from 120 to 134 degrees; and the unhybridized p orbital is used for pi bonding with the an O atom in the resonance contributor, and thus having partial pi bonding with both O atoms in the resonance hybrid).
-
Originally posted by Flying grenade:
Hi Ultima, may i ask you,
in continuation to this https://www.dropbox.com/s/obq34agw4who7ay/forum%20ss.PNG?dl=0 (pg 19 of this forum thread) ,would ring strain affect geometric isomerism?
i've googled, and found these images, with 3 C atoms [http://images.tutorvista.com/cms/images/44/example-of--cis-trans-isomerism.png] ,
5 C atoms [http://wps.prenhall.com/wps/media/objects/340/348272/Instructor_Resources/Chapter_05/Text_Images/FG05_20-17UN.JPG] ,
and 6 C atoms [http://mcat-review.org/cis-trans-ring.gif]
, that can exhibit geometric isomerism, hence unsure how *big* ring is needed to exhibit geometric isomerism? ( in regard to your advice *This ring is too small to have geometric isomerism about the alkene group.* )
Rings can have geometric isomerism. Alkenes can have geometric isomerism. But alkenes as part of rings can have geometric isomerism only if the ring is large enough. Exactly how large? Try asking your school teacher or private tutor (for Singapore JC students reading this), see if he/she knows.Bonus : Why are trans alkenes usually more stable than cis alkenes? For alkenes as part of rings, are cis alkenes more stable or trans alkenes? How large (in terms of no. of atoms that make up the ring) must the ring be for cis alkenes to be as stable as trans alkenes? Below that number, which geometric isomer is more stable? Why? Above that number, which geometric isomer is more stable? Why? Try asking your school teacher or private tutor (for Singapore JC students reading this), see if he/she knows.
-
Originally posted by Flying grenade:
Another example of debye forces?
YJC/2016/JC2 block test
https://www.dropbox.com/s/lt8n85mfhkwn2ma/20160407_104726.jpg?dl=0
Cher say its pd-id forces
All 3 types of van der Waals are present, but the predominant (ie. most extensive) van der Waals is permanent dipole - induced dipole Debye van der Waals forces. -
Originally posted by Mrworry:
When compound C6H5OCOCH2CH3 is heated with NaOH (aq), why it results into Phenoxide ion and CH3CH2COO- instead of a phenol and CH3CH2COO-?
When compound C6H5NHCOCH2CH3 is heated with NaOH (aq), why it results into C6H5NH2 and CH3CH2COO- instead of a (C6H5NH-) (Na+) and CH3CH2COOH?
1st qn : because OH- is able to deprotonate phenol (google up on how the conjugate base phenoxide ion is stabilized by having its negative formal charge delocalized by resonance over the O atom and the ortho, para, ortho C atoms of the benzene ring).2nd qn : because carboxylic acid is far more acidic than phenylamine (which is in fact, far more basic than it is acidic ; to deprotonate phenylamine, you need an extremely strong base such as LDA, which is beyond the A level syllabus), and hence, OH- deprotonates carboxylic acid instead of phenylamine.
MrWorry, no offence, but from your questions, it's evident that your fundamentals (in what makes something acidic or basic) is severely lacking. If you're taking your A levels 6 months later this year in 2016, you might wish to engage private tuition asap.
To add 2 points to MrWorry's questions :
#1 - You're expected to be aware that you have to use excess NaOH(aq), heat under reflux, to carry out hydrolysis or ester or amide groups. Since NaOH is in excess, deprotonation of acidic groups such as COOH or phenol will occur.
#2 - To *fully* understand the reaction (including the exact structure of the products, including protonation/deprotonation), you need to draw out (or at least view) the full curved-arrow electron-flow reaction mechanism. If your school teacher or private tutor don't want to teach you (I teach my BedokFunland JC students these mechanisms), google them out yourself.
To add a further (and last) point about hydrolysis of ester and amide groups, while at A levels we simplify it to call it "acidic hydrolysis" versus "alkaline hydrolysis", at Uni level, it is more correctly termed "acid-catalyzed hydrolysis" versus "base-promoted hydrolysis". Can you figure out why?
-
Originally posted by Flying grenade:
from wiki : Hydrobromic acid has a pKa of −9, making it a stronger acid than hydrochloric acid, but not as strong as hydroiodic acid. Hydrobromic acid is one of the strongest mineral acids known.
why hydroiodic acid stronger than hydrobromic acid stronger than HCl?
e.g. 1 mol of HBr vs 1 mol of HCl, both are strong acids, so dissociate completely to give 1 mol of H+ (or H3O+) correct?
why some acids that donate 1 mol of H+, some acids are stronger?
for HI and HBr, is it because H+ more readily dissociated ? but both strong acids dissociate completely ? so idk
"Completely" actually means "almost completely". The Ka of HI(aq) > HBr(aq) > HCl(aq). But since HCl (the weakest of the 3 acids) is already 99.999% dissociated, at A levels we simplify matters by rounding it off to 100% and say that HCl is a "strong acid", and hence for aqueous (ie. water) solvent, you can say HCl, HBr and HI are all "strong acids" and "dissociate completely".Nonetheless at the same time, based on what you've learnt at A levels about acidic strength, you must still be able to also state that based on H-X bond dissociation enthalpy and stability of X- conjugate base, HI is still considered a stronger Bronsted-Lowry acid than HBr, which is still stronger than HCl, in terms of position of equilibrium and thermodynamic favorability.
So for A level purposes, state and explain both perspectives (ie. they're all strong acids which dissociate completely, but HI is still stronger than HBr which is still stronger than HCl). The distinction of acidic strengths becomes more pronounced and significant when using other solvents instead of water (ie. beyond the A level syllabus).
-
Originally posted by Flying grenade:
thank you !
F.R.S cannot occur at benzene because it's electrons are delocalised by resonance along the benzene ring and hence forming a pi electron cloud, hence forming strong bonds between the C atoms? can help check proper phrasing, thanks !
heat(not only u.v.?) can also initiate the breaking of cl2 molecule to form cl radicals?
First of all, don't use acronyms. Secondly, who told you this?"F.R.S cannot occur at benzene because it's electrons are delocalised by resonance along the benzene ring and hence forming a pi electron cloud, hence forming strong bonds between the C atoms."
That is not a correct explanation of why free radical substitution occurs on the side-chain of benzene rather than along the benzene ring itself. I don't want to spoil the fun for you guys, go ask your school teacher or private tutor why, see if he/she knows.
Yes, heating instead of UV light can also initial the presence of free radicals. However, your syllabus wants to test you to see if you know that UV light can accomplish this, and is more appropriate under certain industrial conditions (ie. where for various reasons you do not want high temperatures at this stage of the reaction), so you must still write "UV light" for the conditions in the A level exams, to generate free radicals.
-
Originally posted by Flying grenade:
my school has a organic chemistry reagents and conditions , and distinguishing/observation test quiz.
a screenshot provided :https://www.dropbox.com/s/kl3gjmpfyp9twlj/sss.PNG?dl=0
how is cold kmno4 be able to test presence of alkenes?
is it because only alkenes can react undergo mild oxidation with cold kmno4, and purple kmno4 is decolorised?https://www.dropbox.com/s/ge44xkvdzqt37kf/ssss.PNG?dl=0
both H2SO4 and NaOH can be used with KMnO4 ?
what's the diff by using AlCl3 and FeCl3? the similarity is it both have empty orbitals?
Yes, correct.Yes, correct. Temperature is the most crucial factor, not pH.
Yes, correct, plus the fact that both just need to accept 1 more (dative) bond from 1 more halide ion (from a halogen molecule) to achieve a stable octet, which is why it has high propensity to do so, consequently generating a unipositive formal charged X+ halonium electrophile, that may be readily attacked by the weak nucleophile benzene ring.
-
Originally posted by Flying grenade:
Hi sir, can enquire your help on this,
https://www.dropbox.com/s/5gyb09xdknrbuuz/20160329_154547.jpg?dl=0
how to see if bond is more polar? (Option c)
Is polarity of bond due to the concept of electronegativity difference? Another factor is asymmetry or symmetry of molecule?
Option C : why a more polar O-H bond (compared to N-H bond) will result in stronger H bonding?
Polarity of bonds are determined by charges and dipoles, which are turn determined by #1 - electronegativity, #2 -formal charges, #3 - resonance delocalization, #4 - hybridization of orbitals. -
Originally posted by Flying grenade:
Hi Ultima, may i ask you,
https://www.dropbox.com/s/1ytc70wi31pu11c/20160329_152652.jpg?dl=0
Will this molecule have pi e- cloud? Nt sure because not uniform atoms, alternating N and P atoms instead of only C atoms
Can Si potentially form at Y? Theoretically, it could right? As period 3 element has energetically accessible vacant d orbitals. Then N atom can donate dative bond to Si?
In this case, is it P is more likely than Si, because form single bond more natural than dative bond ah (idk)??
If it were dative bondings, formal charges must be indicated. Diagram did not indicate formal charges.Pi electron cloud present, but not uniformly distributed like benzene.
-
Originally posted by Flying grenade:
What are the factors affecting strength of Hydrogen bonding?
Is one of the factors electronegativity difference? E.g. H bond between 2 molecules which the H atom bonded to a F atom, is stronger than the H bond between 2 molecules which H atom bonded to O atom?
Are there anymore factors?
Formal charges affect strength of H bonding.And extensiveness (ie. number) of H bonds present are even more important than strength of individual H bonds.
-
Originally posted by Mrworry:
Hi
Can we differentiate HOC6H4CH2NH2 and H2NC6H4CH2OH with LiAlH4 in dry ether? thanks.
First of all, both your analytes' formulae have a typo error, if there are 2 substituents on the benzene ring, it's -C6H4-, not -C6H5-.No, you can't. No group in either molecule can react with LiAlH4. You have to use K2Cr2O7 (but not KMnO4), heat, and observe for color change, which will occur for only 1 of the analytes.
For an alternative method, if both analytes are present in ppt form, only 1 will dissolve with NaOH(aq).
-
Originally posted by Mrworry:
I noticed my mistake (about using LiAlH4) and edited the question. Why with K2Cr2O7, but not KMnO4 though ? How about Benzamide and ethanamide with Br2, FeBr3 + heat? thanks tq
Write out the products generated when you use K2Cr2O7, versus for KMnO4, then you'll see why.Yes, that might work, but the yield will not be high due to the deactivating amide group (which withdraws electrons by both induction and resonance). Although this isn't a synthesis question, but if the yield is minimal, any observation wouldn't be clearly visible, making this a poor method to distinguish between the two analytes.
An alternative and better method would be to use LiAlH4, followed by alkaline aqueous iodine.
-
Originally posted by Light5:
http://papers.gceguide.com/A%20Levels/Chemistry%20(9701)/9701_w13_qp_43.pdf
http://papers.gceguide.com/A%20Levels/Chemistry%20(9701)/9701_w13_ms_43.pdf
http://papers.gceguide.com/A%20Levels/Chemistry%20(9701)/9701_w13_er.pdf
In Q6 part f(i) , i dont understand 2 things.
First of all, i dont understand why G and H are not similar to F...if we rotate both the left and right hand side of the molecule, shouldnt we get F.
Secondly, how does rotating right hand side of J by 60o clockwise make ot identical to F as suggested by the examiner report. And why is Chirality of Left hand carbon of both G and H opposite to that of F.
After all this, how to solve f(iii) ie drawing the fourth optical isomers.
Is this related to R,S configurations or is this something else...as to where can i read more about this?
If you could explain this i detail, i would be grateful..thanks.
This is the toughest question in the paper for 2 reasons : because it's slightly beyond the A level syllabus, and also because it's extremely difficult to visualize (solely using imagination) the rotation of 3D structures.Going beyond the syllabus, ie. at Uni level, the correct way to approach such questions is to assign priorities to the 4 different groups based on the Cahn–Ingold–Prelog priority rules, and concordantly label each of the 2 chiral C atoms present as either R (Rectus) or S (Sinister). The 4 optical stereoisomers would then be (2R,3R), (2R,3S), (2S,3R) and (2S,3S), where (2R,3R) & (2S,3S), and (2R,3S) & (2S,3R), are the 2 sets of enantiomers, while (2R,3R) & (2S,3R), (2R,3R) & (2R,3S), (2S,3S) & (2S,3R), and (2S,3S) & (2R,3S), are the 4 sets of diastereomers.
Alternatively, or simultaneously, if you can obtain (or make for yourself) a 3D 'ball-&-stick' model of the molecule, and rotate each end, it'll help you to better visualize and solve this question. Of course, you won't be allowed to bring in such tools or toys in the A level exams, so you'll have to settle with using your hands : orientate your fingers (using both hands) in a tetrahedral geometry, with 1 finger towards you (the wedge), 1 finger away from you (the hash or dash), and 2 fingers (1 from each hand) neither towards or away (the normal bonds), then slowly rotate your hands. While simultaneously mentally keeping track of which finger representing which group of atoms, *and* the priority of each group (if you're assigning R vs S configuration to help you answer this question). Not an easy task, unfortunately.
You could say this question is unfair to be asked at A levels. But it's a bell-curve anyway, so since it's unfair for everyone, it's arguably still fair. Such questions are intended for only the top few % of the A level cohort to be able to answer correctly, otherwise too many people would score A grade. Regardless of where you're ranked in the cohort, exam-smart students would always skip such time-consuming questions first, complete the rest of the paper, then go back to such 'skipped' questions last. It'll be penny-wise-pound-foolish to waste 15 min struggling on this 3 marks question, then find out at the end of the exam that you only have 3 min left with 15 marks of much easier questions left unanswered.
Originally posted by Light5:Lets assume that i know how to assign R and S priorities
To assign these, the lowest priority group has to be oriented away from us..in this case, the lowest priority group is the Hydrogen atom but the problem is that on the Left Hand Carbon atom for each of the structures, the H-atom is shown to lie on the plane rather than pointing out or into the plane...so assuming that we know how to assign R and S, how can we get past this issue?
By visualizing the structure vividly in 3D space using your imagination, than slowly rotating (not just the single sigma bond, but move the entire structure) it in your mind, until the lowest priority substituent, is pointing away from yourself. And try using your fingers (orientated in a tetrahedral geometry, eg. left hand 3 fingers in a trigonal pyramidal tripod stand, right hand's forefinger representing lowest priority facing up, before rotating it till it's away from you) to help.Also see these videos :
https://www.youtube.com/watch?v=auphHXYK8tE
https://www.youtube.com/watch?v=kFD6hzLseVs -
As a chemist, it's annoying to read stuff like, "Singapore recorded its highest temperature in a decade this month, as the mercury soared to 36.7 deg C". - http://www.straitstimes.com/singapore/wednesday-hottest-day-in-a-decade
Helloooo, due to its toxicity, the use of mercury in thermometers has been phased out. Ergo, the journalistic use of "mercury" to mean "temperature" is outdated. Hmmph.
Did you know? Research studies have shown that dentists generally suffer from lower IQ compared to medical doctors, as a result of cumulative brain damage from long-term exposure to mercury in their dental work. Needless to say, if you've mercury teeth fillings, you've been suffering from cumulative brain damage all these decades. How do you think the phrase "Mad Hatter" came about? Be sure your children don't suffer the same tragic fate (especially tragic because it's totally preventable, if only more people knew).
-
Originally posted by Flying grenade:
What are the causes of amphoterism?
Tried to research online, but to no avail ! The closest relevant info i can find is 'Amphoterism depends on the oxidation states of the oxide' from wiki! But still, this piece of info doesnt help much!
Prior to researching i only know Al2O3 is amphoteric,
but after researching now i know Al(OH)3 , Zn (OH)2, Be(OH)2 are also amphoteric species.
I encountered a qn asking if SrO is amphoteric.
We only learnt and memorised that AL2O3 is amphoteric. So how can we determine which species is amphoteric is a qn asked?
A level shortcut : covalent = acidic, ionic = basic, hence partial covalent partial ionic = amphoteric.BedokFunland JC (ie University) level : to understand identify and understand why & how a species is amphoteric, and why the A level shortcut works (it's not an explanation, but merely an indirect indicator), you first need to understand why & how an acidic species is acidic, and why & how a basic species is basic, and you *must* draw out the curved-arrow electron-flow reaction mechanisms. It's too much detail to go into on the forums, and my BedokFunland JC students can ask me to teach them these mechanisms, while the rest of you can go ask your school teacher or private tutor if interested.
So do you think strontium oxide qualifies to be amphoteric? Btw, amphiprotic is a subset of amphoteric, but not vice-versa. Don't confuse these terms. Go ask your school teacher or private tutor if interested.