BedokFunland JC's A Level H2 Chemistry Qns (Part 2)
-
Originally posted by Flying grenade:
All aminos acid are polar right ? since alpha carbon is singly bonded to 4 diff groups of atoms
including glycine too, which has 2 H atoms bonded to alpha carbon
please kindly check page 36 of this thread where i uploaded my drawing of proline !
Amino acids are not just polar, depending on pH and specific amino acid, they can even be dinegatively anionic, uninegatively anionic, zwitterionic, unipositively cationic, or dipositively cationic.All of these species (including the zwitterion) is even more 'polar' than a 'polar molecule' (the term implies overall neutral, and usually with no formal charges present), due to formal charges present and (other than the zwitterion) hence also ionic charges present.
-
If you're itching to do some calculation involving water, calculating the pKa of H2O (at 25 degrees Celsius) is more relevant to the syllabus. Try calculating it out yourself before you google the answer. (Hint : you'll first need to calculate the molarity of pure water, before you can calculate the pKa of water).
As practice for drawing full structural formulae (all you JC students, school teachers and private tutors reading this), try drawing out the full displayed structural formula for the following species, showing all lone pairs, bond pairs, formal charges, oxidation states, and dative bonds (if any), for the following structures. Challenge yourself to draw them out yourself first, before you google out the answer to check.
ClO
ClO2
Cl2O
Br2O3
Br2O4
Br2O5
Cl2O6
Cl2O7
S2O4 2-
S2O5 2-
S2O6 2-
H4P2O7
H5P3O10
KHF2
[ClF6]+
[AsF6]-
[XeF]+
[SbF6]-
[XeO2F]+
[XeOF3]+
[C6F5Xe]+
[(C6F5)2BF2]-
HN3 (not NH3)
[N5]+
IH5O6
[H2I2O10] 4-
[I2O9] 4-
((Methyl)2SiO3)3
C3H3N3O3
P4O6
P4O7
P4O10
P4O6S4
[P3O9] 3-
[P3O10] 5-
[P4O12] 4-
[SiO4] 4-
[Si2O7] 6-
[Si6O18] 12-
B4Cl4
B9Cl9
B2H6
B4H10
B5H9
B5H11
[B6H6] 2-
[B12H12] 2-
[B2(O2)2(OH)4] 2-
[B5O6(OH)4]-
[B4O5(OH)4] 2- -
Originally posted by Flying grenade:
why polymer, then cannot have geometric (cis-trans) isomerism? there's double bond within polymer
In protein polypeptides, because the lone pair on the N atom is delocalized by resonance, hence the peptide bond in the resonance hybrid actually has partial double bond character, which means sigma bond rotation is restricted by the partial pi bond, which means that whichever geometric isomer (usually the more stable trans isomer) the protein polypeptide was originally generated with (ie. during the intra-cellular translation process involving ribosomes, mRNA and tRNA), that will be the permanent geometric isomer for that particular peptide bond in the protein polypeptide. -
Originally posted by Flying grenade:
page261 cstoh advanced guide d orbital splitting
page 222 2nd print George chong inorganic chem book d orbital splitting
page 450 K.S. Chan physical inorganic chem book
george chong's and K.S. Chan's book show energy of d* orbitals are of higher energy than ground state 3d orbitals, in a free metal ion
cstoh's book shows the splitting causes some orbitals to be of less and more energy compared to 3d isolated orbitals
Here is your answer. Always cross reference across multiple sources, *plus* think critically for yourself : ask yourself which version should be more correct, and why? Apply yourself and your own free-will with self-respect and self-responsibility, instead of blindly following dogma (be it academic, family, cultural, societal, ethical, moral, religious, etc).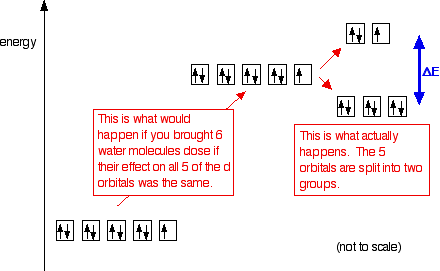
http://www.chemguide.co.uk/inorganic/complexions/colour.html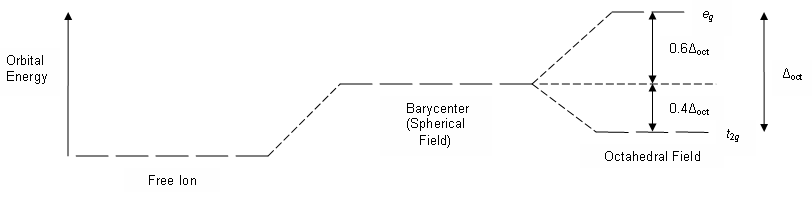
https://en.wikipedia.org/wiki/Crystal_field_theory#Crystal_field_stabilization_energy -
5.00g of an anhydrous Grp 2 metal nitrate loses 3.29g in mass when heated strongly. Identify the metal.Originally posted by Molestermannn69:Hi, I have a question for chemistry and read you can post them here?
5.00g of an anhydrous Grp 2 metal nitrate loses 3.29g in mass when heated strongly
2M(NO3)2 => 2MO+4NO2+O2
What is metal M?
Magnesium
Calcium
strongtium
barium
Originally posted by Flying grenade:Originally posted by UltimaOnline :
I'll leave it to Flying Grenade (don't disappoint me) to show you how to solve it.
5.00g of an anhydrous Grp 2 metal nitrate loses 3.29g in mass when heated strongly
2M(NO3)2 => 2MO+4NO2+O2
What is metal M?
Flying grenade's suggested solution
mol = mass/mr
let mr of M be x
ηM(NO3)2 = 5/(x+124)
ηMO=1.71/(x+16)
since stoichiometric ratio of M(NO3)2 to MO is 1:1
we can equate ηM(NO3)2 = ηMO
solving two equations simultaneously, we obtain x=40.1 (3sf)
Metal M is Calcium.

Correct. Well done, Flying grenade.Fyi, my own working is [ 5g / [ 3.29g / (molar mass of 4NO2+O2) ] x 2 ] - [ (molar mass of NO3) x 2] = 40.1g
But your working will of course get full marks as well. Cambridge only marks the final answer, as long as your working is reasonable, you've some creative freedom to work it out as you wish.
-
Originally posted by Flying grenade:
can write cold, alkaline kmno4 (aq)?
or must write cold, kmno4 (aq), NaOH(aq)?
for formation of diol fron alkene
can write acidified K2Cr2O7 (aq), heat
or must write H2SO4 (aq) , K2Cr2O7 (aq), heat?
my sch say must write the compound out
In the A level exams, the reagents and conditions for each reaction, should be written in exactly the same way that either CS Toh, or Chan Kim Seng, or George Chong writes them (ie. above & below the equation arrow) in the reaction equations. Cambridge will accept any of these alternative ways of writing the reagents and conditions. All JC students are advised to closely follow these 3 sources, over their own school lecture notes. -
Originally posted by Flying grenade:
page 130 cs toh advanced guide
for electrolysis of brine, why when Hg is used as cathode, Na(s) is liberated, instead of H2(g)?
redn potential of H+/H2 =0.00V shld be more +ve than Na+/Na
from data booklet, no Na+/Na redn potential value, but have for Li+/Li, which is very -ve.
is it becos if there is no electrode(as in without the Hg electrode) , the Na(s) want to deposit but no where to deposit? (and hence H2 is liberated instead? lolol)
for electrolysis of Na2SO4, the H+ at the cathode come from water H2O uh?
Mercury thermodynamically (both enthalpically and entropically) stabilizes the reduced sodium metal in amalgam, and also on further condition that a concentrated (not dilute) solution is electrolyzed. -
Originally posted by Flying grenade:
pg 130
for electrolysis of H2SO4, why H2SO4 slowly become more concentrated?
for the anode reaction, Cs toh provided 2 equations
1st equation, more water form as product
2nd eqn, water as a reactant, then form O2 as product
At cathode, 4 H2O used (to supply the 4 H+), and O2 + 2 H2O + 4 OH- byproduct generated (not written in CS Toh's eqn, since he used 4 H+ instead of 4 H2O as reactant).At anode, 4 H2O used (to supply the 4 OH-), and 2 H2 + 4 H+ byproduct generated (not written in CS Toh's eqn, since he used 4 OH- instead of 4 H2O as reactant).
Hence, total H2O used = 8, but total H2O generated = 2 + 4 = 6.
Therefore, overall balanced redox equation is 2 H2O(l) --> 2 H2(g) + O2(g), and the solution becomes more concentrated (as H2O is electrolyzed away).
Note that Singapore JCs will insist you use H2O as the reactant for both cathode & anode, instead of CS Toh's H+ and OH- respectively.
Cambridge will accept both versions, unless the solution being electrolyzed is strongly acidic or strongly alkaline; which is why Singapore JCs have decided it's safer to instruct JC students to just always use H2O as the reactant.
And if you can't figure out why, or which cathode & anode equations are acceptable or not acceptable in such electrolytic setups of strong acidity or alkalinity, go ask your school teacher or private tutor.
-
Several years ago on SgForums, I've already posted on the toxic effects of food cooked in aluminium foil. And yet, it's still commonly used in food preparation today, one of many aspects of 'modern civilization' contributing to increasing toxicity in today's diet and life.
https://sg.style.yahoo.com/why-you-shouldnt-cook-with-aluminium-foil-124406855.html
-
Here in Singapore, you can get your hands on the world's most powerful toothpaste, which pitiful Americans in the US of A are denied access to. Google up on the biochemical properties of Novamin, which pharma giant GSK eagerly paid Sg$200 million to hostile takeover. And now Singaporeans can get what Americans can't.
-
Originally posted by Flying grenade:
Hi Ultima, please take a look at above qn, from 2007 (can't edit on same post cos can't find the cursor using phone hence cant type on the post above with pic
part V)
i understand can illustrate using the pair of same amino acids, but tcher instructed us to illustrate using pair of different amino acids to make it more challenging
https://www.dropbox.com/s/md4v22dtowaymp1/20160714_100436-1.jpg?dl=0
Question :
https://www.dropbox.com/s/amfgl1ifmblcm99/20160714_101102-1.jpg?dl=0 drawn three lys-ser R group interactions
do we have to draw full displayed formula when showing R group interactions?
need to show the linear H bonding between the R groups? is my drawing of (3) acceptable?
from the Qn, HSA is a globular protein, and has a roughly spherical shape in water, 67% of the amino acids are incorporated into an α-helix
if need show linear H bonding , then lys and ser is one shorter and one longer than the other,
if both R groups must originate from the same vertical starting position, then showing linear H bonding is quite difficult
Thanks, Ultima
You only need to fully display out the part of the R group which is directly responsible for the R group interaction. The rest of the R group can be condensed or skeletal.As far as possible, always show linear H bonding. Yes, your drawing of (3) is acceptable (except it can be improved, see next sentence below).
Regarding originating from the same vertical starting position : your drawing is actually misleading and incorrect.
Don't put a hypen between "lys" and "ser", it implies you wrongly think these are 2 directly adjacent amino acid residues (which would only be peptide bonded to each other, not H bonded by R groups).
The way you drew it out, from the R groups from same 'starting position', is incorrect and misleading. Because the amino acid residues with the R group interactions will be many residues apart, and because the polypeptide chain is non-linear (think of the polypeptide chain as curving and twisting convolutedly all over the place), hence you should have drawn the 2 amino acid residues as originating from different 'starting positions' on paper, either facing each other, or (more realistically) at an angle away from each other. See CS Toh's diagram on the bottom of page 381 (Advanced Study Guide).
Lastly, don't forget you're attempting to translate a 3D structure onto 2D paper. Each C-C bond is bent in 3D space, not linear. On 2D paper, it may not always be possible to illustrate perfect linearity (for all H bonds in all molecules), just do your best to reflect this. Cambridge will be reasonable.
Originally posted by Flying grenade:if say, lys and ser is side by side joined by a peptide bond, is there H bonds between the R groups?
Usually not, due to steric factors. R group interactions are usually, and most importantly, between non-adjacent amino acid residues. If the only R group interactions are between adjacent amino acid residues, then there would be no meaningful tertiary structure, which is what gives proteins its biological function. In which case, proteins would be useless, and you would have died even before you were born.Now you understand why your drawing is misleading and incorrect? And your school teacher didn't point out this error?!? Or worse, your school teacher taught the entire class this wrong way?!?
Originally posted by Flying grenade:you mean no H bonds between adjacent lys and ser due to steric factors, not Lys and ser cannot exist side by side due to steric factors right?
ultima, pls take a look at pg 38 of this thread to see 2 more posts I've posted
Yes, obviously. Lys-Ser and Ser-Lys amino acid residues certain do exist. But R group interactions do not usually exist between adjacent amino acid residues, and are far more important and meaningful (in terms of tertiary structure enabling biological function) when they occur between non-adjacent amino acid residues, as is mostly the case.
Originally posted by Flying grenade:Yes Ultima, exactly. Whole class was taught wrongly. Ultima, that's why i am very very thankful ,of you, helping us type out, and more importantly, Elaborate out All(small and big, important and the nitty gritty ) the Details, and Most Importantly, teach and correct us to learn the correct and right education.
my only regret is i didn't discover BFJC earlier.
I Feel Very Frustrated and sad that I've discovered so much that I've learnt throughout my life, is wrong. Happy because at least i discover it.
i am very thankful that i receive education from BFJC, one of the only true educators and education.
Another important skill I've acquired from BFJC is to learn to suspect and question and double check if something is taught wrong, and i seek clarification via books, online, and forums.
some people don't even know they're learning the wrong things(like me in the past, receive without critically evaluating and discerning what I've learnt ), and once they discover,they'll be angry and happy.
for me, I haven't been doubting Chem stuffs I've learnt until this year, it's late, i wished i was more inquisitive and a critical thinker last time, but better late than never
Very good, but at the end of the day, you still need to bring home to bacon. Being physically incarnate, the highest pinnacle of success necessitates success in all aspects of your life.This includes academia. Even if you've now grown to understand Chemistry more deeply than your school mates, but at the end of the day, if you still fail to perform under examination conditions, and still fail to score an A grade (or whatever grade is to your personal satisfaction, for whatever goal), it means you've failed and disappointed yourself (not others), in that you've not lived up to your maximum potential.
For your own sake, don't disappoint yourself. Get the grade you know you can, for this year's upcoming A levels.
-
In an earlier post, I mentioned that the sterics do not allow significant tertiary interactions between adjacent amino acid residues (as wrongly taught by your school teacher, and therefore unsurprisingly, probably many other Singapore JC teachers as well).
To understand this more clearly, use google images to check out alpha helices and beta-pleaded sheets (ie. the 2 most common secondary structures).
Alpha helix : https://www.google.com.sg/search?q=alpha+helix&tbm=isch
Beta-pleated sheet : https://www.google.com.sg/search?q=%CE%B2-pleated+sheet&tbm=isch
Notice that in both secondary structures (for different sterical reasons), the R groups of adjacent amino acids are oriented in 3D space (ie. sterics) away from each other, making R group interactions between adjacent amino acids difficult, minimal or downright impossible.
-
Originally posted by Flying grenade:
page 148 cs toh advanced guide
example 6, at bottom of page
PCl5 (g)《》PCl3(g) +Cl2 (g)
yes i understand cstoh's explanation, and understand less PCl5(g) would dissociate
noted that Kp is not affected by changes in pressure (because partial pressure or in other cases, concentration, the partialP/conc , of both reactants and products increases/decreases by same amount right? )
but i thought of what I've learnt ,read , and done questions before,
when P decrease, eqm position shift left as predicted by LCP, wouldn't conc PCl5 increase?
See see see?!? Again, I bet your school didn't teach you properly. This is one of the all too common misconceptions suffered by Singapore JC students.When pressure is changed (by changing the volume of the container), the molarities and partial pressures of the reactants and products change, hence because Kc & Kp doesn't change (since temperature doesn't change), and Qc & Qp now changes, hence position of equilibrium shifts, so as to allow Qc & Qp to equalize with Kc & Kp, to re-establish equilibrium.
In other words, position of equilibrium shifts BECAUSE Kc & Kp doesn't change. Geddit? U think ur school mates truly understand this concept / topic, when even u urself are confused?
Originally posted by Flying grenade:page 148 cs toh advanced guide
example 6, at bottom of page
PCl5 (g)《》PCl3(g) +Cl2 (g)
yes i understand cstoh's explanation, and understand less PCl5(g) would dissociate
noted that Kp is not affected by changes in pressure (because partial pressure or in other cases, concentration, the partialP/conc , of both reactants and products increases/decreases by same amount right? )
but i thought of what I've learnt ,read , and done questions before,
when P decrease, eqm position shift left as predicted by LCP, wouldn't conc PCl5 increase?
You COCONADEN!!! (more pleasant, organic vegan/vegetarian version of cockanaden)Becoz [PCl5] increases (as you mentally worked out), *THATS WHY* CS Toh wrote "less PCl5 dissociates (at equilibrium)"!!! Again, as posted earlier, this shifting of position of equilibrium is because Kc & Kp value did NOT change (since temperature didn't change).
-
Originally posted by Flying grenade:
i see.
i think despite NH3+ grp have 3 H , possible to form H bonds but it will be more thermodynamically favourable to form ion dipole interactions rather than H bonds
Do you realize (no you don't, and neither do 99.9% of Singapore JC students), that depending on the exact ion and molecule (ie. case by case), the so-called ion - permanent dipole interaction (don't be lazy, there are 2 types of ion - dipole interactions) may actually be a hydrogen bond (enhanced by the formal charge present)?Positive formal charge = electron-withdrawing by induction, enhancing strength of H bond donated. Negative formal charge = more electron-rich, enhancing strength of H bond accepted.
True ion - permanent dipole interactions are inorganic (eg. Na+ and Cl- with H2O solvent) rather than organic (eg. amino acids), because you also need to consider the geometry (eg. tetrahedral) of the molecule. So understandably, Singapore JCs don't teach all these coz they (fairly enough) know Singapore JC students will be confused by all these.
-
The following are the interactions between ion and ion, ion and polar molecule, ion and non-polar molecule, polar molecule and polar molecule, polar molecule and non-polar molecule, non-polar molecule and non-polar molecule, arranged from strongest to weakest, ceteris paribus.
ion - ion (ie. ionic bonding)
ion - permanent dipole
ion - induced dipole
permanent dipole - permanent dipole
permanent dipole - induced dipole
instantaneous dipole - induced dipole
-
Originally posted by Flying grenade:
SORRY CORRECTION
R GROUP NH2 is more basic than Alpha NH2 group in the FIRST PLACE, and hence, will definitely be protonated first, followed by the alpha NH2 group.
i gt good smart qn haha
lp of e- of N atoms of R group of Arginine are delocalised by resonance. since the extra stability conferred by resonance,
how would we know if the NH2 R group would be more willing in accepting a proton, and hence more basic, compared to the alpha NH2 group?
https://en.m.wikipedia.org/wiki/Arginine
this is the issue that was bugging my mind just now, but couldn't gather my thoughts fast enough.
the two N atoms of the R group of Arginine has partial double bond due to delocalisation of electrons by/due to resonance.
will the / how do we know if, either of the R group N atoms, is more stable, and hence, may or may not readily accept proton compared to Alpha N atom of Alpha NH2 group, and hence may or may not be more basic than the alpha NH2 group,
or the R group NH3+ may or may not be more acidic than alpha group NH3+ as the tendency to be protonated is more or less
Obviously the R group imine is more basic than the alpha amine, especially in Arginine. Because now there are 2 reasons, not just 1. Induction (electron-donating by induction R group) and resonance (the conjugate acid is stabilized by having its positive formal charge delocalized by resonance over 2 N atoms).Your confusion lies in thinking the lone pair of the R group N atom is delocalized *before* protonation, and hence less available to accept a proton. Nonsense.
First of all, only the R group amine's lone pair is slightly delocalized by resonance before protonation, but the R group imine's lone pair isn't, and it is the R group imine which is protonated (not the amine). Thereupon, the R group amine's lone pair then becomes extensively delocalized, in order to allow the positive formal charge on the conjugate acid to be effectively delocalized, across both N atoms.
-
Originally posted by Flying grenade:
for delocalisation of electrons to occur, the adjacent atom with respect to the atom containing the unhybridised p orbital with a lone pair of e- must have an empty unhybridised p orbital?
Unhybridized p orbital, yes. Empty, no. -
Originally posted by supercat:
Hi, I have a question regarding 2014 AJC Prelim, paper 2 Qn 5.
Regarding option C, when 4 methylbenzoic acid reacts with Br2(aq), why won't C8H8O2Br2 form? The positions of CH3 (2,4 directing) and COOH (1,3 directing) will support bromination right?
Because the benzene ring of para-methylbenzoic acid isn't sufficiently activated to attack the Br2(aq), since CH3 is only electron-donating by induction and hence weakly activating, while COOH is electron-withdrawing by both induction and resonance, and is hence moderately deactivating.Even without the electron-withdrawing COOH group, methylbenzene still isn't sufficiently activated to be able to attack Br2(aq). You need anhydrous Br2 with AlBr3 or FeBr3 Lewis acid catalyst for that.
And if free radical substitution of the methyl group is desired, exposure to UV light is required.
-
Originally posted by supercat:
Thank you very much, I understood your explanation. For the same question (2014 AJC Prelim, paper 2 Qn 5), for option B, there won't be bromination then? Since it is COOCH3, I infer that it is also electron withdrawing but still not sufficient for bromination.
For 2014 AJC Prelim, paper 3 Qn 2ciii, may I ask how is T formed? What type of reaction could possibly occur to form the alkene by-product? At first I thought that it could be SN1 mechanism since it is secondary alkyl halide. But it will not be able to form an alkene, especially with the removal of O, since O is needed for attacking the alkyl halide.
For 2014 AJC Prelim, paper 3 Qn3d, I had trouble deducing Geraniol. I could deduce N,P,Q and R, but I wonder why Geraniol doesn't have 2 terminal alkene. Since heating geraniol with excess KMnO4 will give N and P, and we see that 2 carbon has been removed, so why is there no 2 terminal alkene present? 2 CO2 could be removed. Instead, the product is a diol. Are diols colourless gases?
Perhaps Supercat has difficulty taking a photo of the qns or uploading them. But as Flying Grenade said, everyone asking H2 Chem qns here, do try to take a photo and upload the qns whenever possible, for the sake of sharing and helping other JC students here, coz not everyone has access to all the Prelim papers.2014 AJC Prelim, paper 2 Qn 5 : Correct, ester group is electron-withdrawing by both induction and resonance, hence the benzene ring is deactivated, no halogenation via electrophilic aromatic substitution occurs.
2014 AJC Prelim, paper 3 Qn 2ciii : E2 reaction occurs in competition with SN2. While the Singapore A level H2 Chem syllabus simplifies the matter of whether nucleophilic aliphatic substitution (ie. SN1 vs SN2) or elimination (ie. E1 vs E2) occurs as simply a matter of solvent (ie. aqueous vs ethanolic), but in practice (ie. real-life and Uni level Chem), all 4 compete against each other, and there are other factors involved in the 4-cornered fight : SN1 vs SN2 vs E1 vs E2.
While E1 and E2 mechanisms are not required for the Singapore A level H2 Chem syllabus, but nonetheless as evidenced by the AJC Prelim paper (and all Singapore JC Prelim papers and significantly tougher recent Singapore-Cambridge A level H2 Chem papers since 2010), it pays for students gunning for A grade to have a deeper understanding of Chemistry beyond the basic A level H2 syllabus. Which is the advantage that H3 Chem, Olympiad Chem, and BedokFunland JC Chem students have over H2 Chem only students.
2014 AJC Prelim, paper 3 Qn3d : You're right that the 2 missing C atoms are eliminated in the form of CO2(g). But you're incorrect that 2 terminal alkene groups must therefore be present in geraniol. Because if that were the case, how would heating geraniol with acidified KMnO4 yield N and P and CO2(g)? The given formula of geraniol precludes the acidic hydrolysis of ester or amide group as a possible explanation.
Concordantly, the student is expected and required to deduce and elucidate that the functional group in question (that results in 2 C atoms eliminated as CO2) is either the =CHCH2OH group, or the =CHCH= group, either of which is oxidized by KMnO4 with oxidative cleavage into ethandial, which is further oxidized to ethandioic acid, which is further oxidized to 2 moles of carbonic(IV) acid, which exists in equilibrium with, and hence decomposes into, 2 moles of CO2(g) + H2O(l), with thermodynamically favorable positive entropy change, and with the position of equilibrium shifting to the RHS as predicted by Le Chatelier's principle (since CO2(g) leaves the aqueous reaction mixture).
“Once you eliminate the impossible, whatever remains, no matter how improbable, must be the truth.� - Sherlock Holmes (Parody1 Parody2 Parody3) -
Originally posted by supercat:
Hi Flying grenade, prelim papers are available on the internet, I googled them online.
http://score-in-chemistry.weebly.com/2014-jc-prelim-papers-and-solutions-from-internet.html
^ AJC 2014 prelim
For E1 and E2, I searched them online. So I take it that nucleophilic sub occurs together with elimination? And SN1, E1 favours sec/tertiary compounds while SN2, E2 favours pri/sec compounds? In this case, then I see nucleophilic elimination "similar" to normal elimiation reactions (dehydration) in the sense that a C=C bond is formed? From my understanding, alcoholic solvents tends to promote elimination while (aq) tends to promote substitution. Does acidic/alkaline conditions play a role too?
Btw, why are methanoic acid and ethanedioic acid so special? They are the only 2 unique carboxylic acid that can be further oxidised to CO2 and H2O. Are there any reasons for this? Is it because of their structure?
Whether pH needs to be acidic or alkaline, depends on the specific elimination reaction. Ideally, you (ie. all JC students) should be able to draw out the elimination mechanisms, instead of blindly memorizing about it, which will enable you to effortlessly decide if acidic or alkaline conditions are required. Tip : Draw the elimination of HX from alkyl halide, and the elimination of H2O from alcohol. One requires alkaline pH, the other acidic, for reasons that will be obvious once you draw out the mechanisms. And ideally, you (ie. all JC students) should be able to draw both E1 and E2, to see the relationship with SN1 and SN2.Because of the high O to C ratio for these molecules, allowing ease of further oxidation, and hence more positive oxidation potentials.
-
Originally posted by Flying grenade:
can individual amino acids (residues ) lys and ser undergo neutralisation?
what is the R group interaction between lys and ser in a protein? is it ionic interaction as a result of neutralisation between the R groups? or is it H bonding? or is it indeed ionic interactions, but for reason idk
can neutralisation between R groups occur within the protein?
First of all, "neutralization" is an O level term. At A levels, the only time you're allowed to use this term is "enthalpy of neutralization". For physical chemistry, you must state "Bronsted-Lowry acid-base reaction". For organic chemistry, you must state "proton transfer reaction". Using "neutralization" will get you penalized at A levels.Ser's R group is an aliphatic alcohol, non-acidic OH group. Lys' R group is amine/ammonium. Hence R group interactions between Lys and Ser is hydrogen bonding.
To your next question, the answer is "obviously", but also "you're thinking it wrongly". If you have a deeper understanding of Chemistry, you'll realize that the protonation or deprotonation is not actually between particular R groups and other particular R groups (eg. "Asp transfers proton to Lys", etc), but each R group protonation/deprotonation actually occurs with the aqueous solvent environment in the biological organism (eg. human body tissue fluids). In other words, solvent catalysed Bronsted-Lowry acid-base proton transfer reactions.
-
Originally posted by Flying grenade:
Thanks, Ultima !!!
omg , difficult qn
online no S2- and S22- structure
Alevel 2014/p2/qn2dii
ultima can help check my drawing of disulfide ion, S22- , if it is correct
https://www.dropbox.com/s/5e2za7uiwdmge1x/20160719_135608.jpg?dl=0
S can expand octet
the answer provided by publisher no additional 2 electrons
Wrong lah. S2- is 4 lone pairs, 0 bond pair for that 1 S atom. S2 2- is 3 lone pairs, 1 bond pair, for each S atom with a uninegative formal charge, hence dinegative ionic charge.
Originally posted by Flying grenade:Thanks Ultima savior, got it
i was careless, publisher's answer is indeed correct
why my drawing wrong? its plausible since S can expand octet, and its more thermodynamically favourable to form double bond
1stly, how are you going to achieve a dinegative ionic charge without 2 uninegative formal charges? And how are you going to achieve 2 uninegative formal charges for the 2 S atoms, unless each S atom has 3 lone pairs and 1 bond pair?2ndly, you're wrong, it's actually more thermodynamically favorable for period 3 elements to form more single bonds instead of double bonds, which is why CO2 has double bonds while SiO2 has single bonds, another classic example being nitrogen versus phosphorus molecules, etc. And do you know the correct explanation for this? Go ask your school teacher or private tutor, don't ruin the fun for others on the forum by posting the answer here.
-
Originally posted by supercat:
http://imgur.com/oMkVJ6s
Is this the correct mechanism for E1 & E2?
Can see that this reaction needs alkaline medium. So that there would be alkoxide formation which can attack the molecule. Correct??
For LiAlH4, why does it only reduce ketones/aldehydes, but for H2 & Nickel catalyst, it reduces all alkenes, ketones, aldehydes?
Yes, your E1 and E2 mechanisms are correct. But if you're using alkoxide ion as the base, then the correct reason why you need alkaline pH is to prevent protonation of your alkoxide base, so it can carry out the elimination.Alkene is a nucleophile, as is also the H- ion from LiAlH4 and NaBH4, hence no reaction occurs (they're not gay). In contrast, H2(g) with nickel catalyst is able to provide both H+ and H- (as consequence of H2 adsorption-reaction-desorption with the transition metal catalyst with partially filled d orbitals), and hence can hydrogenate the alkene nucleophile / base.
-
Originally posted by Flying grenade:
pg 253 cs toh advanced guide, the other examples with [CuCl4]2- , for Co-ordination number 4, sq planar, are all affected by Jahn-Teller effect right?
The Jahn-Teller distortion is particularly notorious (and you're required by the A level syllabus to memorize this example) for the deep blue tetraamminecopper(II) coordination complex ion. All other 4 ligand coordination complexes, eg. tetrachlorocuprate(II), must be assumed to be tetrahedral, unless hints (eg. cis and trans isomers exist for a 4 ligand coordination complex) are given by the question that it's square planar. -
^_^