BedokFunland JC's A Level H2 Chemistry Qns (Part 2)
-
In the 1st experiment, what's cool is that the slug fragmented into 2 equally lethal parts *in mid air* before reaching the target, *both* fragments subsequently penetrating the solid metal armor. In the 2nd experiment, what's cool is that upon hitting the solid metal armor, the slug fragmented into many dozens of molten metal shrapnel fragments, radiating spectacularly sideways (imagine if you were standing just beside where the slug hit), as illustrated by the explosions of the 2 filled soft drink bottles to the sides of the metal armor. Chemistry note : a "carbide" is a compound composed of carbon and a more electropositive element (eg. a metal), and isn't a metal per se, as erroneously stated by the YouTube video host. But let's not fault him, and appreciate his video making and sharing efforts instead. He can leave the Chemistry checks to us... makes you appreciate taking H2 Chemistry more and more everyday, don't it? ;Þ
-
[Medical Science] - An adenovirus causes obesity in humans and animals
https://www.wired.com/2016/12/mysterious-virus-cause-obesity/
In the visitors comment below the article, Mindbreaker posted :
There is too much money in treating chronic conditions. DARPA funded something that can cure all sorts of viruses, but it just disappeared into a black hole never to be seen again. It was called DRACO (Double-stranded RNA Activated Caspase Oligomerizer) and it killed every virus thrown at it.
This adenovirus causing obesity is not a new revelation. This has been known for years. The first tip-off was that the obesity epidemic acted exactly like a spreading infection through the population. It followed the same geographic patterns. There is almost nothing else it could have been. There are at least 3 adeoviruses that likely cause or at least contribute to obesity, adenoviruses #5, #36, and #37. And possible several more that are more difficult to study. And Firmicutes have also been implicated.
It is not just obesity, lots of conditions have been linked to infections. The Wikipedia page that listed them was removed. Fact is, the big pharmaceutical companies have lost billions when it is discovered that a bacteria causes something they were earning billions to treat. Perhaps you have heard that they discovered that ulcers are caused by a bacteria? That woke up the pharmaceutical industry. That hurt them. Then it was discovered that a bacteria causes lower back pain and damage. After that, amazingly it became very hard to get antibiotics. Doctors are getting in trouble for prescribing antibiotics for anything that is not proven to be caused by bacteria...they don't want another accidental cure. They say it is to stop the development of supper bugs. But most of the antibiotics used in the US are used on millions and millions of animals everyday...just to make them grow faster...nothing to do with illness. If there are super bugs being born that is likely where they are coming from. The reality is that they can develop new antibiotics very easily. They avoid it. And there were things that killed bacteria before antibiotics that still work on "super bugs", but you never hear about them.
The evidence for staph bacteria causing diabetes has not been followed up even though the evidence was more than trivial. They gave staph infections to rabbits and they got diabetes. Coincidence? I doubt it. You can have a staph infection and not even know it. Infections can be invisible.
Most of the chronic conditions we have are because of these infections be they viral, bacterial, fungus or other. Did you know human cytomegalovirus (HCMG) causes more cases of serious birth defects than Down Syndrome, Fetal Alcohol Syndrome, Spina Bifida, or HIV/AIDS? But I bet you have never heard of it. Why? Because the thing is everywhere. They can't isolate the infected from the uninfected. And they don't bother to create a cure because it is likely the condition slowly kills you...very slowly, that means lots of symptoms to treat for decades. 60%-70% of us are infected, and the older you are the more likely that someone will infect you. Hard to separate the affects of old age from the effects of infections the immune system is just loosing the long battle against. HCMG is a herpes virus. They never get eliminated, they just do damage for decades (without vigorous treatment). And it is spreading fast. Rates have gone up dramatically since the 1970s. I don't think it is an accident that Americans' life expectancy is starting to move downward despite really impressive progress against cancer. Doctors know many cancers are caused by viruses. They call them oncoviruses. They currently acknowledge that they cause 17.8% of human cancers, but it probably is a far larger proportion. Perhaps 50% or more. I find it interesting that the success they have had recently is due to revving up the immune system. Coincidence that that might be a way to fight tough viruses in your body? I don't think so.
We are all carrying around dozens of infections. If they do not kill quickly, or produce clear identifiable symptoms, doctors say it is "normal". Well, it isn't. Before people could go all over the planet and things were not spread all over, the average person almost certainly had far fewer infections cruising around in their bodies. I bet you do not even know that sweets do not cause cavities; that it is a bacteria, just one of over 100 types of bacteria in your mouth. And there are tribes who don't have this highly contagious infection. No, it is not "normal" it is an infection that has gone everywhere and infected almost everyone on the planet, but was probably very rare and limited to one small area in the past. But with nothing done about it...its everywhere. Believe it or not, but they have a cure. But so many ridiculous barriers were erected that it never got enough funding to survive the tests. The FDA asked them to do ridiculous things like test only on adults with no teeth. I don't know about you, but if I was down to 2 or 3 teeth I would not give up those last few. Who then would have no teeth? And did you know that tooth problems are linked with heart disease. Those plaques in your arteries are just like that bacterial plaque on your teeth. The bacteria that causes dental carries is a different one than the one that causes gum disease, rots your jaw and infects your arteries and your pancreas.
The biggest impediment to recognizing the cause of disease in our day is the "weakness" stigma. The, "well just control yourself", "use good hygiene"...sort of blame stuff. I think virtually all mental illnesses are caused by infections including anxiety and depression. And much of the rest caused by heavy metals, other toxins and nutritional deficiencies. I believe genetics has been greatly exaggerated...just one more "weakness" story to make others feel superior and invulnerable.
In the Southern US people got the reputation for being lazy. But it was hookworm. Just sucks the energy out of you. Fortunately people finally saw the light and made the changes necessary to infrastructure and cured the infections, and that lousy thing is gone. Still in Africa and other places infecting half a billion people. People who no doubt are still looked down on as lazy, and worthless.
It is entertaining to think it is a conspiracy, that pharmaceutical companies prefer treating chronic people to curing people and obstruct the development of cures. But it probably is not. It is probably just a defect with the way medicine is developed in the US, the economics of it. Turf protection, and barriers to market entry do exist, how much that has played into policy and harmed the pubic is an open question.
-
Originally posted by cienhan:
hello ultimaonline,
when heated with carbon, azurite produces copper, carbon dioxide and steam as the only products. write a balanced eqn for the reaction of azurite with hot carbon. azurite is Cu3(OH)2(CO3)2
thank you in advance.
Decomposition :OH-, OH- [proton transfer] O 2-, H2O.
CO3 2-, CO3 2- [elimination] O 2-, CO2, O 2-, CO2.
Cu2+, Cu2+, Cu2+, O 2-, O 2-, O 2- as ionic solid + CO2, CO2, H2O as molecular gases.
Redox :Cu2+, Cu2+, Cu2+, O 2-, O 2-, O 2- + 1.5 C [electron transfer + covalent bond formation] Cu, Cu, Cu as metallic solid + 1.5 CO2 as molecular gas.
Hence overall balanced equation is :1 Cu3(OH)2(CO3)2 (s) + 1.5 C (s) ----> 3 Cu (s) + 3.5 CO2 (g) + 1 H2O (g)
This is the BedokFunland JC way, seeing clearly the mechanisms involved (proton transfer, elimination, electron transfer, covalent bond formation) in the type of reactions occurring (decomposition, redox) and recognizing the underlying thermodynamic motivation (positive entropy change) for it. -
^_^
-
^_^
-
^_^
-
^_^
-
^_^
-
To all JC students, here's a BedokFunland JC teaser for your entertainment :
The N atom in both amines and amides have the same no. of lone pairs and bond pairs. Yet the hybridization of the N atom in both types of molecules are different. See if you can figure out why before googling out the answer.
-
If you're burning anything or have your stove on, always make sure the windows are open. I could explain to you the underlying chemistry involved (ie. as carbon is a relatively non-electronegative element, a uninegative formal charge on the sp hybridized C atom in the major resonance contributor of carbon monoxide results in an unusually strong Lewis base, which thus behaves as a competitive inhibitor ligand to nucleophilically attack the electrophilic Lewis acidic Fe atom in the haemoglobin molecule in red blood cells generating the kinetically and thermodynamically stable carboxyhaemoglobin with a much larger Kstab value than oxyhaemoglobin... etc) but just be sure you remind your family and friends to be mindful.
https://sg.news.yahoo.com/six-teens-found-dead-garden-party-germany-192344411.html
-
Both medical doctors (Dr Daniel Neides and Dr Zubin Damania) may furiously disagree with each other, but I agree with both of them, ie. they both make valid points. As with almost everything in life, it's not a simple case of right or wrong, black or white. It's more a matter of pros and cons, risks versus benefits, and making informed choices (philosophical musing : or can there really be such a thing?).
Dr Daniel Neides : http://www.cleveland.com/lyndhurst-south-euclid/index.ssf/2017/01/make_2017_the_year_to_avoid_to.html
Dr Zubin Damani : https://www.facebook.com/ZDoggMD/videos/10154799367477095/
Btw, Dr Zubin Damania hates Dr Mehmet Oz
-
Originally posted by hoay:
Activation energy is not affected by increasing temperature.It is the catalyst which decreases the Ea but when we say increasing temperature increases the proportion of molecule having energy greater or equal to the activation energyit seems that Ea is linked to temprature. Please explain.
Say you have 10 employees, but as a stingy CEO, you underpay them with unethically low wages. One night after work, the 10 of them try to enter a high-class nightclub, but only 2 of them have enough money to afford the $100 entrance fees and enter, while the remaining poorer 8 of them can only wait pitifully outside the club.Some years later, after having a near-death experience, your perspectives as a CEO have changed, and now you decide to pay all your 10 employees generously. Now all of them have enough money to pay the $100 entrance fees to enter the nightclub. Happy ending.
Originally posted by hoay:It means that if we increase the temperature the the demand or the parameter for Ea is increased also ....but i am unable to deduce catalyst here...the CEO or salary?
Catalyst isn't a factor in this story. This story is about increasing their pay so they've enough money to enter, ie. more employees now have enough money (energy) to afford the entrance fee (activation energy).For a catalyst story, it would be a near-death experience for the nightclub boss, causing him to decide to lower the entrance fee (activation energy) required to enter (for reaction to occur).
So EITHER increasing the emplyees' pay (molecules' energy), OR reducing the entrance fee (activation energy) required, would help to increase the % of employees who can enter, hence speed up the rate of the reaction.
You can of course, do both. But for A levels, Cambridge will test your students on each factor separately, not simultaneously.
-
[Medicine] - If you go to medical school, you will be stressed. Bigly.
http://www.kevinmd.com/blog/2017/02/go-medical-school-will-stressed-bigly.html
[Medicine] - Female medical doctors told to get out of the way during emergencies as patients nearly die on planes
-
^_^
-
Originally posted by keefay:
Ascorbic acid (vitamin C), an essential nutrient for humans, is an organic compound containing carbon, hydrogen & oxygen. when 1.000g of ascorbic acid was completely burnt in oxygen, 1.500g of carbon dioxide and 0.405g of water were formed. Calculate the empirical formula of ascorbic acid and determine its molecular formula if its relative molecular mass is 176.
Hi UltimaOnline, how should i start the question? i tried to form the equation of the combustion of a carboxylic acid but i couldn't get the answer :(
Thank you so much! :)
That's because Vitamin C isn't a carboxylic acid.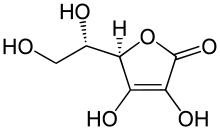
From moles of CO2 generated, find moles of C in sample to be 3.4091x10-2 mol, ie. 4.0909x10-1 g.
From moles of H2O generated, find moles of H in sample to be 4.5x10-2 mol, ie. 4.5 x 10-2 g.
Hence mass of O in sample = 1.00 - (4.0909x10-1 + 4.5x10-2) = 5.4591x10-1 g, ie. 3.4119x10-2 mol.
Moles of Vitamin C = 1.00 / 176 = 5.6818x10-3 mol.
Hence mole ratio of VitaminC to C to H to O is 5.6818x10-3 : 3.4091x10-2 : 4.5x10-2 : 3.4119x10-2, which simplifies to 1 : 6 : 8 : 6.
Hence molecular formula of Vitamin C = C6 H8 O6
No prob, KeeFay! :)
-------------------------------------------------
BedokFunland JC Challenge Qn
Cambridge can ask as an A grade H2 A level exam question, "Identify the most acidic H atom, hence or otherwise, explain why Vitamin C is acidic."
(Interested JC students go ask your school teacher or private tutor for the answer)
-
Originally posted by keefay:
hi ultimaonline,
for S2O8^2-, the OS of S is +6. i figured it out through drawing the structure but i don't get the same answer using O level method. why is it so?
thank you so much! (:
Edited to add in italicsBecause the O level method is just an average value and makes simplified assumptions about common OS values for various elements which aren't always correct in context, while my BedokFunland JC formula for OS (which only the best few Singapore JC teachers are familiar with) is superior in that you can use it to calculate the OS of individual atoms in individual resonance contributors.
In more complicated molecules and ions such as the S2O8 2- ion, you *must* use my BedokFunland JC formula to arrive at the correct OS. A recent Singapore H2 A level exam question in which Cambridge asked for the OS of a certain atom, also requires my BedokFunland JC formula to arrive at the correct OS.
BedokFunland JC FTW! ;Þ
-
A disgrace to us Chemists! :(
-

O-ethyl S-[2-(diisopropylamino)ethyl] methylphosphonothioate
Kim Jong-Nam assassinated by VX nerve agent : Malaysian CSI police labs
https://sg.news.yahoo.com/vx-nerve-agent-found-kim-jong-nam-face-010253670.html
"One of the two women suspects who remain in custody fell ill after the brazen killing, with police saying Friday she had been vomiting."
It's clear by now that the Vietnamese and Indonesian women 'assassins' are themselves innocent victims, made use of by the manipulative North Korean espionage agents, then mercilessly and cruelly sacrificed to take all the blame and face execution or life imprisonment for the assassination.
Inside the shadowy North Korean espionage agencies accused of killing Kim Jong-nam
http://news.asiaone.com/news/asia/inside-shadowy-north-korean-espionage-agencies-accused-killing-kim-jong-nam -
Yo 2017 JC1 student Lockheed2000.
This is an interesting question that was asked by Cambridge in a TYS qn, yet a proper understanding of how the lamp works actually goes beyond the H2 syllabus.
For the simplified version that will suffice for the H2 syllabus (which being oversimplified for A level purposes, is unfortunately inaccurate and misleading in several ways) :
Neon is already gaseous, while sodium is solid, under standard conditions. Hence upon switching on the lamp, the neon gas is ionized first, producing a red glow, while it takes some time for the sodium metal to be vaporized then ionized, then producing the yellow-orange flow of sodium. Na(g) having a less endothermic 1st ionization energy compared to Ne(g), ionizes more readily, and hence being present in a larger quantity, its yellow-orange glow thus predominates.
(Beyond H2 syllabus, for those interested)
But the above simplified A level H2 version is misleading in several ways. Firstly, the ionization itself doesn't produce the glow. The glow occurs when the atoms (whether neon or sodium) transit from a higher energy state, to a lower energy state, in the process emitting electromagnetic radiation or photons of a specific frequency corresponding on that element's emission spectrum (that sodium has a brighter glow than neon, is actually due to their different emission spectrums, rather than the misleading A level explanation given above). This actually happens after, and is separate from, the ionization process.
When the lamp is turned on, the electricity applied ionizes and oxidizes the Ne(g) at the anode, and the Ne+(g) cations are accelerated by the applied electric field toward the cathode, in the process colliding with Ne(g) atoms, which transfer their electrons to the Ne+(g) cations. The Ne(g) atoms (having lost an electron during the collisions), ionize into Ne+(g) and accelerate toward the cathode, while the Ne+(g) cations (having gained an electron during the collisions), become reduced to Ne(g) *and* return to a lower energy state, emitting a photon of light of neon's characteristic frequency (ie. red glow) corresponding to neon's emission spectrum.
The above process generates the heat required to first vaporize the Na(s) to Na(g), and then ionize the Na(g) to Na+(g) at the anode, and thereafter, the same collision process described above with Ne(g) and Ne+(g), then takes place with the Na(g) and Na+(g), resulting in the emitting of photons of light of sodium's characteristic frequency (ie. bright yellow-orange glow) corresponding to sodium's emission spectrum. In practice, inter-elemental collisions between the neon and sodium atoms and ions do occur as well.
Advice to JC students : always first understand the simplified A level concepts first (A levels is a transition bridge between O level child's play, and the real Uni level science), and then only if you're interested and able to handle it, delve into the more correct Uni level details.
Originally posted by Lockheed2000:Dear UltimaOnline,
I am a J1 this year, and I've just started my first Chem tutorial on Atomic Structure. However I'm stuck on this question. Can you help me with it?
Give a brief explanation of the following observation in terms of ionisation energy.
Orange street-lamps contain sodium with a small amount of neon, and light is produced when gaseous atoms are ionised in an electric field. It is observed that when the street-lamps are turned on, a red glow characteristic of neon is first emitted and the orange glow of sodium predominates after a time.
-
Yo Carychidestar 2017 JC1 student.
70 cm3 = unreacted O2 + CO2 (H2O is liquid at rtp)
Since 20cm3 = unreacted O2, hence 50cm3 = CO2
Since 80 cm3 of O2 used per 10 cm3 of hydrocarbon, hence (5 + y/4) = 8, ie. y = 12
Hence molecular formula of hydrocarbon is C5H12
Originally posted by Carychidestar:Hi Ultima.I have a question that I would like you to answer.
"When 10cm cube of a gaseous hydrocarbon were exploded with 100cm cube of oxygen,the residual gas occupied 70 cm cube.After shaking these residual gases with aqueous sodium hydroxide,the final volume was 20 cm cube.All volumes were measured at room temperature and pressure.Calculate the molecular formular of the hydrocarbon."
Does the residual gas includes the excess,unreacted oxygen?(CO2+O2+H2O)
The final residual gas is (O2+H2O)?
Since CO2 reacts with NAOH.
I'm not sure how to solve the 'residual gas' type of combustion analysis.I can solve the normal ones though.
Ans is C5H12
I got 5 for C but I have no idea how to get H...
I just compare the volume ratio and mole ratio
-
For the past few decades, medical science has warned that saturated fats cause cardiovascular disease, but little was known or mentioned about trans fats. In recent years however, medical researchers from around the world, including Cambridge University, has found that on the contrary, there was no link between saturated fats and cardiovascular disease, but instead there was a strong link between trans fats and cardiovascular disease. So it turns out saturated fats was the innocent wrongly maligned guy, and trans fats was the evil hidden twin brother carrying out the murders.
BedokFunland JC's A level H2 Chemistry Qns :
What are saturated fats, and why are they called "saturated"? What are trans fats, and why are they called "trans"? Suggest how trans fats could contribute to cardiovascular disease. -
Originally posted by Carychidestar:
Thanks for the reply.
' 10cm3 of a hydrocarbon Z were exploded with 100cm3 of oxygen in excess.The volume of the gaseous products was 80cm3 but was decreased to 40 cm3 when these products were bubbled through aqueous akali.All volume were measured under the same conditions of temperature and pressure.What is the molecular formula of Z?'
How do I solve problems like this does not give the conditions(temperature and pressure)?
I am not able to determine if steam is also part of the residual gases.
I only know that CO2 is 40cm3.
AND
A completely off-topic question: When do you think the A level assessment books are going to come out?(Updated according to new syllabus)
Due to syllabus changes,the books that are on the shelves of popular bookstores currently aren't really useful.
Just based on your own experience.
Thanks in advance.
You don't need temp and pressure, coz such qns will not require the use of PV=nRT. H2O is always taken to be liquid under standard conditions, unless the question implies that H2O is gaseous under the question's non-standard conditions.Oxygen used = 60 = 10(4+y/4) hence y = 8, ie. hydrocarbon is C4H8
The 2016 TYS is already out, but since the latest 2016 paper is old syllabus, hence you will *not* be able to get any TYS updated to new syllabus before the 2017 A level exam. This has always been the case for every pioneer batch of syllabus change. No worries, doing the old syllabus TYS is still useful, and your school teachers will inform you of the parts of the syllabus that have changes, as well as give you adequate practice for the new content not in the old syllabus exam papers.
-
Originally posted by hoay:
Which statement is correct about a reaction for which the equilibrium constant is independent of temperature
A the equlibrium constant for both the forwar and reverse raection do not vary with temperature.
B the enthalpy change is zero.
C the activation energies for both forward and backward raction are zero
D there are equal number of moles of reactants and products.
why A is wrong? I have replaced rate constant with equlilibrium constant ....in this case choice A will still be wrong?
For your modified question, both A and B are correct. For the original Cambridge question, only B is correct. Even if a reaction is neither endothermic or exothermic, increasing temperature will increase both the k-forward and k-backward rate constants, but the magnitude of increase will be equal, meaning that the Kc-forward and Kc-backward values will be unaffected.Originally posted by hoay:So Rate constant and equilibrium constant are different here for this case.
Can you present an exmaple of this type of raection?
Cambridge was just testing the conceptual understanding of students, *IF* such a reaction exists.In practice, for enthalpy change of a chemical reaction to be zero, is theoretically possible but extremely rare (in contrast, after taking entropy change into account, for Gibbs free energy change to be zero, ie. neither endogernic nor exogernic, at a particular temperature, is extremely common, eg. freezing / melting of water at 0 degrees C).
One such possible reaction (and in fact the only case within the A level syllabus), for enthalpy (and in this case, also entropy and Gibbs free energy) change of reaction to be zero, would be the conversion of 1 enantiomer to the other, eg. an anion as nucleophile replacing the same anion as leaving group via SN2, or via SN1 (which cheats a little by using overall reaction rather than individual elementary step-wise reactions).
There are other rare examples, but which are beyond A levels and need not be considered, and will not be asked by Cambridge.
Originally posted by hoay:For a gaseous system...increasing pressure increases the rate of forward reaction as there are more colliding particles but why does it increases the rate for a reverse raection..do we assume that the increase in pressure also increases the no of particles of product side also...in case of 2nd stage of Contact process.
Increasing pressure of a system by decreasing its volume, increases the molarities of both reactants and products, hence increasing the rates of both forward and backward reactions.But if the no. of moles of gaseous reactants vs products are different, the magnitude of increase in rates of forward vs backward reactions will be different (this can be shown mathematically as the stoichiometries of the reaction determine the Kc and Qc formulae), hence position of equilibrium will concordantly and consequently shift towards the side with less moles of gas (if pressure is increased by decreasing volume).
-
Originally posted by Jasmine855:
Hi, I’m a JC graduate who has just receive her A level results.
I got As in GP, 4H2s and O level HCL, with B in PW. I have been considering applying for a science course in either NUS or NTU ,such as chemistry,pharmacy,life sciences etc.1) Medicine?
I ruled out medicine as I thought it’s out of the question, as I didn’t expect to get nearly straight As + my lack of good CCA records &consistent CIP. However, seeing my results, my mum asked me to try for medicine, saying that I might have chance of getting in. I could only apply to NUS since I didn’t take the BMAT for NTU.The main question would be of course:whether I have a passion in serving as a doctor. To be honest, I don’t really have a clear idea. I enjoyed learning the various body systems and diseases in Sec 4 Bio but have never seriously considered to enter medicine. My interest in Bio is reduced in JC as we started learning micro things instead of the human body.
Qn: What do medicine students really study? Would it be back to Sec4 Bio kind of syllabus but of higher level?I joined Red Cross as a CCA in JC because I don’t really have other choices and I was slightly interested in the various first aid equipment like which is used to treat what. Throughout the 1.5 years, I’ve only volunteered a few times outside of school as a first aider(as I was struggling with JC academically). Seeing other fellow passionate first aiders I was inspired by their commitment. I also learnt the joy of serving as the casualty thanked me after I helped them bandage their wounds. However, I also discovered a problem with myself, that is I’m afraid to talk with strangers, let alone offering support/comfort for the casualty,using words. Although I care about them, I don’t dare to show it. I have no idea why I have grown into such a shy person <- this is what I heard from quite a few people.
My other concern would be my mediocre CCA record, which is quite important to enter medicine apart from grades right? I have no CCA positions throughout my life and also no stellar CIP records(as I mentioned-have to keep mugging in jc). I don’t think I still have chance for medicine =_= Actually I have applied for two attachments to hospitals so as to enrich my experience in the healthcare sector and also beef up my record, sadly both were rejected,one by JC and one by RMG. Through this experience, I’m convinced that I wouldn’t fit as a doctor, so I stopped considering medicine.
Besides, I’m also worried about the stress of being a medical student and subsequently as a doctor. I have experienced various weird symptoms in my body due to stress since secondary school. From bruxism leading to Temporomandibular Joint Disorders and sensitive teeth, stomachache before exams, to pimple outbreak before As, I am thinking what else would happen to me under the stress in medical school. Even though I have taken steps to relieve stress by exercising or whatsoever, I realised I’m a person who likes to overthink and give myself stress. If so, I think I wouldn’t survive in medicine.
All in all, do you think I should go for pharmacy instead of medicine? So that
1. I can still help others in a healthcare sector and take time to improve my social skills without killing a life if I were to enter medicine.
2. I can face less stress compared to being in medicine??
(Tbh, I can only visualize myself as a researcher making contribution towards science behind the scene instead of being a front-line person, although I haven’t had any true research experience lol)2) Bio vs Chem
As mentioned earlier, I liked Bio less in JC because the syllabus is full of micro-level stuff and also cuz of its rigidness (like there’s tons to keywords to memorise). I enjoyed Chem in JC more probably because it’s easier to learn. In my case, should I apply for chem course instead of bio(life sciences/biological science)?Pardon me for the long post as I didn’t really have someone to talk to…Your help is deeply appreciated!!
You should go ahead and apply for Medicine as your 1st choice. Even with straight As, chances of entering Medicine is very low, so just give it your best shot.
Regardless of your initial motivation for entering Medicine, even if it's not as selflessly noble as some other applicants, what matters the most is the good you can contribute to society and to humanity as a medical doctor, regardless of your initial motivations.
There are many, many, many different roles, specialties and subspecialties, that you can take up to contribute as a medical doctor, so don't worry about that now. From frontline ER doctors and surgeons, to family General Practitioners, to backend medical researchers, you'll be able to find a role that best fits you, but all that comes after graduation, so don't worry about that now.
Between Chemistry and Biology...
If you're equally interested in both, go for a double degree (hellishly demanding) or double major (very demanding), or one major and one minor (somewhat demanding), or just one major with no minor (least demanding, and the path chosen by majority of Uni students), in Chemistry and Biology. Otherwise, if you only choose one subject to read in Uni, research and check out professional career pathways for each subject major, before deciding. Besides, at Uni leve, every subject has many specialties and subspecialties, some of which you might prefer Bio, some of which you might prefer Chem. So it's not so straightforward, consider all options carefully with an open mind while applying, and after that, when choosing to accept whichever courses you've successfully applied into.
-
Originally posted by hoay:
In the electrophilic addition reaction of propene in the carbocation the positively charged carbona atom is sp3 hybridized (in propene it is sp2). But the electron geometry is tripgonal planar so it must be sp2 why it is sp3?
During the intermediate, it is sp2. Only upon nucleophilic addition (the 2nd step of the 2-step electrophilic addition mechanism) does it become sp3.
Originally posted by hoay:Actually i was referring to Q.37 of Nov 2009 Singapore A-level. It asks about the change of hybridization from sp3 to sp2 in the reaction internediate.
three reaction were given:
1 CH3-CO-CH3 + CN-
2 (CH3)HC=CH(CH3) + H+
3 C6H6 + +NO2
1 is correct since Carbon joined to Oxygen has 4 eelctron pairs
about 2 the marking scheme says its true also
3 is correct also as in Wheland Intermediate one of carbon is sp3
1 and 3 are correct...2 must be correct also ?
All 3 options are correct.In your earlier post, you asked abt the +ve formal charged C atom of the intermediate, that remains sp2 during the intermediate.
Instead Cambridge is saying, if *either* of the reactant's C atoms transform from sp2 to sp3, then the option is to be considered correct.
For option 2, of the 2 sp2 C atoms of the alkene undergoing protonation, 1 C atom (whose pi bond becomes a new sigma bond) immediately becomes sp3, while the C atom which then bears the +ve formal charge remains sp2, until nucleophilic addition of the Bronsted-Lowry acid's couter anion conjugate base (eg. Cl- ion if HCl was employed as the Bronsted-Lowry acid) occurs, then it becomes sp3 as well in the product.