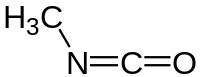BedokFunland JC's A Level H2 Chemistry Qns (Part 2)
-
^_^
-
2017 BedokFunland JC H2 Chemistry Challenge Qn
Draw the full curved-arrow electron-flow multi-step reaction mechanism for the hydrolysis followed by dehydration of (i) aluminium chloride and (ii) silicon tetrachloride, including an appropriate representation of the transition from simple molecular reactant, to ionic product (for i) and giant covalent lattice product (for ii).
Also draw the full curved-arrow electron-flow multi-step reaction mechanism for the complete hydrolysis of magnesium nitride to generate a solid and a gas. The correct stoichiometry must be accurately reflected in the reaction mechanism.
Answer :
BedokFunland JC students can ask me during tuition. Other students can go ask your school teacher or private tutor.
-
^_^
-
Originally posted by hoay:
A gardener fertilizer is said to have a phosphorous content of 30.0% soluble in water.
what is the % by mass of the phosphorous in the fertilizer?
A 6.55 % B 13.1 % C 26.2 % D 30.0 %
Ans ... the balanced reaction is P2O5 + 2H2O............... 2H3PO4
Now 30.0 % of P2O5 (Mr = 142) is 42.6. so mass of phosphorous will be
142g..........62g in H3PO4
42.6..........x g in H3PO4
x = 18.6 g of Phosphorous
since only 30.0% is soluble so out of total mass of H3PO4 (196) only 58.8 is available
18.6/58.8 x 100 = 13.8 %
Is the working correct?
The original Cambridge question is different from the one you paraphrased.For the original question, P2O5 takes up 30% (by mass) of the fertilizer.
Calculate % (by mass) of P in P2O5, ie. (2 x Mr of P) / (Mr of P2O5).
Final answer = (% P in P2O5) x 30%
The "solubility" is just a red-herring, irrelevant to the qn, so just ignore it.
Originally posted by hoay:I got 13.1 % which is the correct answer.
This was from Nov 2003 / CIE / P1. I copied the question as i have it here. Anyway thank you for your prompt answer.
Your original post left out "P2O5" in your question, which changes the question entirely. In future, for all CIE questions, please link directly to the question online, whenever available.Working is (as I explained in my previous post) (30 / 100) x ( ( (2 x 31) / ( (2 x 31) + (5 x 16) ) ) = 0.131 or 13.1%
-
The Chemical Mystery of the Kim Jong Nam Assassination (and why 2 separate women assassins were required) :
https://sg.news.yahoo.com/toxic-mystery-behind-kim-jong-nams-assassination-045311729.html
-
Originally posted by Carychidestar:
Hi ultima,could you help me to solve this question?
I got negative values....
"0.7g of a hydrocarbon K was burnt completely in excess dry oxygen.2.2g of co2 is formed.100cm3 of K required 600cm of o2 gas,measured at same temp and pressure for complete combustion.Determine the molecular formula of K."
Hi Carychidestar,(x + y/4) = 6
[ 0.7 / (12x + y) ] x = [ 2.2 / 44 ]
Solve the simultaneous equations.
-
^_^
-
^_^
-
2 ang moh teenage slackers try out RJC life
-
Originally posted by glitter58:
hi
for Oxidation state questions, when do i use the O level formula and when do i draw out the structure and figure out the OS?
since sometimes both methods give different answers
Depends on the question and the molecule / ion involved, ie. simple vs complicated structure. If in doubt, draw out the structure to work out the OS of the individual atom in the individual resonance contributor (using my BedokFunland JC / University level formula or method for working out OS using structure), then give the more relevant answer (eg. for Organic Chem, in the oxidation of alcohols to carbonyl compounds to carboxylic acid, obviously the O level method, ie. an average OS per element, won't suffice), or if in doubt, give both answers (with explanation of each working). -
Originally posted by keefay:
Hi UltimaOnline, is S in SO3 an expanded octet? Thank you! (:
Yo keefay,There are 7 major resonance contributors for SO3.
For A level H2 purposes, you can take it that the required resonance contributor (ie. the structure that you need to draw if asked in the A level exams) is the one without formal charges, ie. S has 6 bond pairs, 0 lone pairs. Hence in terms of a stable octet, the S atom (in this resonance contributor) has 12 valence electrons, ie. expanded octet.
But as a BedokFunland JC / Olympiad / H3 Chem student, I expect you pple to be cognizant of the other resonance contributors, in 3 of which the central dipositively formal charged S atom has exactly a stable octet with 1 doubly bonded no formal charged O atom and 2 singly bonded uninegatively formal charged O atoms, as well as another 3 resonance contributors in which the central unipositively formal charged S atom has an expanded octet with 2 doubly bonded no formal charged O atoms and 1 singly bonded uninegatively formal charged O atom.
In reality, the resonance contributor without formal charges, though usually taught in Singapore JCs, is actually the most minor of the resonance contributors, in spite of it having no formal charges (can you figure out why? My BedokFunland JC students can ask me during tuition, all other students can go ask their school teacher or private tutor).
Concordantly, all resonance contributors considered, you should be able to elucidate the resonance hybrid. But for A level H2 purposes, unless otherwise specified by the question, it will suffice to draw the simplest resonance contributor without formal charges.
Bonus BedokFunland JC challenge question : identify the orbital hybridization involved in all 4 atoms in the SO3 molecule, and hence describe the exact nature (ie. what type of overlap involving which specific orbitals) of each of the sigma and pi bonds (for the resonance contributors that have pi bonds).
No prob, keefay! :)
-
Originally posted by glitter58:
another question, when butan-2-ol undergoes elimination, how do i know whether the major product is CH3CH2CH=CH2 or CH3CH=CHCH3 ?

There is the kinetic product, then there is the thermodynamic product. With sufficiently high temperatures, you can safely assume the major product is the thermodynamic product*.Internal alkenes are more thermodynamically stable compared to terminal alkenes*, and as such CH3CH=CHCH3 is expected to be the major product over CH3CH2CH=CH2.
(*Why? BedokFunland JC students can ask me during tuition, all other students can go ask their school teacher or private tutor.)
-
^_^
-
Originally posted by flowerd:
Hi! What is meant by "vacant low-lying orbital" for dative covalent bond?
Vacant means empty, unoccupied by electrons.Low-lying refers to being energetically accessible by that element.
Period 1 elements : only 1s orbital is low-lying.
Period 2 elements : 2s orbital, and 2px, 2py and 2pz orbitals, are all low-lying.
Period 3 elements : 3s orbital, and 3px, 3py, 3pz orbitals, and 3d(x2-y2), 3d(z2), 3d(xy), 3d(xz), 3d(yz) orbitals, are all low-lying.
A Lewis acid or electrophile can only accept a dative covalent bond pair of electrons into one of its vacant low-lying orbital (of the acceptor atom).
Originally posted by flowerd:Also I am confused about where to label the bond angles for 3D structures. For example the picture in the link below shows 2 different ways to label. Which one is correct and how do I know where to label the bond angle? :)
http://imgur.com/0KfJUVE
Both are correct and acceptable by Cambridge, because all the bond angles in the tetrahedral molecular geometry are all equally 109.5 degrees. -
^_^
-
Note for H2 Chemistry : it is a non-issue, because Cambridge only cares that you write the symbol for it (ie. 298K), and not spell it out (eg. 298 Kelvins / 298 kelvins / 298 Kelvin / 298 kelvin).
Are there reasons for the discrepancies in absolute temp units - Kelvin vs. kelvins vs. degrees Kelvin?
Before 1968, the units for absolute temperature were described as "degrees Kelvin" or "degrees absolute." After that, the SI system got rid of the idea of "degree" for absolute temperature, so the new unit should apparently be expressed as a "kelvin" (with lowercase k) and abbreviated simply "K" (without the degree sign). Also, official SI conventions suggest that not only should the unit name be lowercase, but it should be pluralized as other units would be: "Il en résulte que la température thermodynamique du point triple de l’eau est égale à 273,16 kelvins exactement, Ttpw = 273,16 K." Or, in English: "It follows that the thermodynamic temperature of the triple point of water is exactly 273.16 kelvins, Ttpw = 273.16 K."
Despite the official SI usage, however, it seems that there are still a variety of conventions in use. Many of the questions on this forum, for example, use Kelvin (with a capital) instead of kelvin in referencing the unit. Also, it appears that the plural usage is somewhat mixed in the physics literature: something like "200 kelvins" occurs, but more rarely than "200 kelvin" or even "200 Kelvin." The NIST guidelines do not list the kelvin as an exception to the normal pluralization rules: "the following plurals are irregular: Singular — lux, hertz, siemens; Plural — lux, hertz, siemens." On the other hand CERN's writing guidelines suggest that there is an exception: "And note that it is always kelvin, even when plural (not kelvins or degrees kelvin)."
Given all of this, here is my question: Is the SI standard actually to pluralize kelvins, as would be suggested from the quotations from the official SI guides above? Is this officially stated anywhere in some standards organization's guidelines? Or, is there some rationale given somewhere for the continued use of plural "kelvin" (as in the CERN guidelines) or even "Kelvin" (with an apparently anomalous capital)?
Or, is it -- as I suspect -- just a failure to treat the kelvin as an actual SI unit, despite the redefinition from "degrees Kelvin" to "kelvins" that happened decades ago? (Perhaps we just dropped the "degree" but effectively still treat it the same way as Celsius or Fahrenheit?)
-
Originally posted by hoay:Q33. http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_w03_qp_1.pdf
but when we increase the temperature the energies of the molecules increases. In statement 3 when it says energies with any given value increases....does it mean that these molecules will NOT be near to Ea?
Yes. Statement 3 says *any* energy value. If you choose a high energy value (eg. Ea), then as you said, increasing temperature will increase the % of molecules with this energy (eg. Ea). But if you choose a low energy value, then increasing temperature will *decrease* the % of molecules with this energy (because increasing temperature provides energy).As an analogy, statement 3 = the no. of pple with any amount of money will increase. If u let any amount = $0, and you increase the wealth of everyone (eg. giving everyone $1million), then statement 3 = "the no. of pple with $0 will increase after giving everyone $1million" is obviously false.
-
Originally posted by hoay:
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_s13_qp_11.pdf
white ppt formed in first reaction that may be AgCl or AgBr or AgI or AgAt.
ppt dissolve in concentrated aqueous ammonia means it is either Cl- or Br-.
But on addition of HNO3 the ppt dissolves....Can you explain what happens here? and why Cl- is present here not Br-? Has this something to do with dynamic equilibrium?
For Q17, since the ppt dissolved in dilute NH3(aq), the ppt must have been AgCl, which re-precipitated out upon addition of acid, because position of equilibrium shifts right : NH3 ligands <~> NH3 (aq) <~> NH4+(aq)To address your question directly, it's Cl- not Br-, because the ppt dissolved in *DILUTE* NH3(aq). If the question used concentrated aqueous ammonia, then both Cl- and Br- could be possible (ie. then the MCQ would be flawed and cannot be answered definitively, unless the color of the ppt was specified).
-
Originally posted by hoay:
Yes i know. But what is most probable energy then?
Probability = Abundance (think gambling at the casino, the more numbers you buy at the roulette table, the higher your probability of winning... of course, if you still lose, you'll also lose more money... so overall in the long run, the 5.26% house edge remains). Most probable = most abundant, ie. the mode (not the median or the mean).Hence if Cambridge asks, "On the Maxwell Boltzmann distribution graph, indicate the most probable energy or speed of the molecules", you have to draw a horizontal line on the y-axis touching the highest peak (ie. most abundant = most probable) of the curve, together with a vertical line from this same highest peak down to the x-axis, which gives you the x-axis value for the most probable energy or speed.
To address your question more directly :
The most probable energy is the energy that is contained by most of the molecules at low temperature. It is different from Ea..is this correct?
Yes, but not at 'low temperature', but at whatever the given temperature is (each temperature will have it's own graph-curve). So if more than 1 temperature-graph-curve (eg. 300K, 350K, 400K) is given in the diagram, then each of these temperature-graph-curves will have its own most probable energy or speed, ie. the x-axis value corresponding to the highest peak on each of the temperature-graph-curves.
It's different from Ea, because Ea is a fixed value for each chemical reaction, that doesn't change with temperature.
-
Originally posted by hoay:
http://papers.xtremepapers.com/CIE/Cambridge%20International%20A%20and%20AS%20Level/Chemistry%20(9701)/9701_s15_qp_13.pdf
Q.8 Why C is not the answer? As the [methanol] will be become constant when the reaction is complete.
Q.28 What is X in this diagram?
Q8. Option C assumes that the basic hydrolysis of ester is a 0 order reaction, but it is in fact, a 2nd order reaction. Hence the correct answer is option A. Option B is characteristic of an auto-catalytic reaction, but neither product of the basic hydrolysis catalyzes the hydrolysis. Option D is just nonsense.Q28. X is the transition state as the C-Br bond is being broken, ie. a C-Br partial bond, a partial +ve charge on the C atom, and a partial -ve charge on the Br atom.
-
Originally posted by hoay:
Another query is from June 2016 /MCQ. I am afraid that this paper is not available in any site on the web.
Methyl isocyanate , CH3NCO, is a toxic liquid.
What is the approximate angle between the bonds formed by the N atoms in a a molecule of methyl isocyanate?
H3C- N=C=O 120 is the answer.
The electron pairs around N are 3 ; there are 2 bond pairsaround N; one is lone pairs. 321....so the bond angle must deviate from the ideal situation 330...120. It would be less than 120, around 109.
Excellent question. There are actually 5 levels to this at-1st-glance straightforward question.At the simplest level, the N atom has 3 electron regions or charge clouds : a single bond, a double bond, and a lone pair. Hence electron geometry is expected to be trigonal planar, ie. bond angle close to 120 degrees.
On a deeper level, the lone pair is expected to have slightly more repelling power, hence the bond angle may now be expected to decrease slightly below 120 degrees.
On an even deeper level, the double bond is expected to have slightly more repelling power, hence the bond angle may now be expected to increase slightly back to approximately 120 degrees.
On a way deeper level, O has the greatest electronegativity, hence the O atom withdraws strongly by induction (ie. through the sigma bond) as well as by resonance (ie. through the pi bond) from the C atom, which consequently itself becomes somewhat electron-withdrawing vis-Ã -vis the N atom, despite N's greater electronegativity vis-Ã -vis the C atom. The effect of which is to reduce the electron density of the N=C double bond, hence the bond angle may now be expected to decrease back to slightly less than 120 degrees.
On a way, way, way deeper level, you should be able to figure out that there is a minor resonance contributor in which the lone pair on the N atom forms a 2nd pi bond with the C atom, and the pi bond between the C atom and the O atom becomes a lone pair on the O atom, ie. +ve formal charge on the N atom, -ve formal charge on the O atom. This is a minor resonance contributor, valid only because of the high electronegativity of O. As such, the lone pair resides in an orbital which is mostly sp2, but has a little unhybridized 'p' character, in order for the slight overlap of unhybridized p orbitals (of N and C) to occur and for slight delocalization of the lone pair to form a partial 2nd pi bond with the C atom, ie. the N=C bond has partial triple bond character in the resonance hybrid. As such, the bond angle may now be expected to increase to above 120 degrees, ie. the N atom is mostly sp2 hybridized, with a little sp hybridized character.
Indeed, experimental evidence has proven the C-N-C bond angle to be approximately 125 degrees, thus concurring with my 5 points of consideration above.
Of course, Cambridge is aware that A level students are not able to consider all these factors to elucidate as deeply as I just did, and hence if it were a non-MCQ question, Cambridge will be reasonable and accept reasonable answers on condition of reasonable rationales. For a MCQ question, Cambridge will (as they did in this MCQ) only provide clear-cut options such as A) 120 degrees B) 109.5 degrees C) 90 degrees D) 180 degrees, in which the required answer is obvious, because the A level student is expected to choose the option which gives the closest answer.
-
One of the toughest questions Cambridge has ever set, is Q6 f ii & iii of the CIE A Level 2013 Nov Question Paper 43.
Another delightfully tricky IQ-type question that Cambridge can be proud of, would be Q5a of the CIE A Level 2009 Nov Question Paper 42.
Go check them out and have fun! ;D
-
^_^
-
BedokFunland JC Challenge Qn on Gases
Sketch the graphs of PV/RT against P, for both an ideal gas as well as a real gas, on the same axes. For the real gas, explain why there is a negative deviation at low pressure, and why there is a positive deviation at high pressure.
-
BedokFunland JC Challenge Qn on Drawing Mechanisms
Images Source : https://en.wikipedia.org/wiki/Methyl_isocyanate
Easy : Draw the curved-arrow electron-flow mechanism for the reaction :
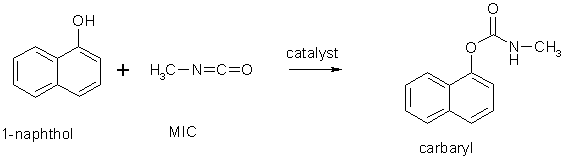
Intermediate : Draw 2 possible curved-arrow electron-flow mechanisms (pericyclic and free radical) for the reaction :

Difficult : Draw the curved-arrow electron-flow mechanism for the extended reaction to generate both products :

(BedokFunland JC students can check their answers with me during tuition, all other students can go check with their school teacher or private tutor.)