Structure of NO2 (using formal charges)
-
Exploring the technique of drawing NO2 and checking with formal charges.
Its a bit difficult to describe without an image.
Total valence electrons = 5 + 2(6) = 17
I ended up deciding between two outcomes.
Case 1
O - N = O, where the unpaired electron is found on the left oxygen.
Case 2
O = N = O where the unpaired electron is found on the N atom
Both cases seeming gives me a formal charge of zero on all 3 atoms. Would there be any reason why we should choose one over the other?
Googling seems to show that case 1 is more widely drawed, but Nitrogen dioxide (apparently) dimerises, which supports case 2.
-
Originally posted by atomos:
Exploring the technique of drawing NO2 and checking with formal charges.
Its a bit difficult to describe without an image.
Total valence electrons = 5 + 2(6) = 17
I ended up deciding between two outcomes.
Case 1
O - N = O, where the unpaired electron is found on the left oxygen.
Case 2
O = N = O where the unpaired electron is found on the N atom
Both cases seeming gives me a formal charge of zero on all 3 atoms. Would there be any reason why we should choose one over the other?
Googling seems to show that case 1 is more widely drawed, but Nitrogen dioxide (apparently) dimerises, which supports case 2.
Both structures you've proposed are incorrect.Case 1 is incorrect because the singly bonded O atom does not have an unpaired electron. If it had one, it would have no formal charge, then because the N atom has a unipositive formal charge, the sum of formal charges would be 1+ and not zero (neutral molecules have an ionic charge of zero). Recall that ionic charge is the sum of formal charges.
Case 2 is incorrect because if your central N atom has 4 bond pairs as well as an unpaired electron, its octet would be violated. Cambridge requires H2 students to state, "Period 2 elements do not have vacant, energentically accessible 3d orbitals to use to expand their octet".
The correct structure is :
O=N-O
with a unipositive formal charge on N, half a lone pair (ie. an unpaired electron) on N, and a uninegative formal charge on O. For physical chemistry, it is advised that students indicate formal charges within the molecules and ions, and (for ions) indicate the ionic charge outside the square brackets (which symbolize summation : of formal charges or oxidation states, to give the ionic charge).
For physical chemistry (but not organic chemistry; never show dative bond symbols in organic chemistry, but instead use curved arrows or normal line bonds, for organic chemistry mechanisms and non-mechanism structures respectively (just be sure you always indicate all formal charges in organic chemistry; do not use square brackets for ionic species in organic chemistry, thus you only need to show formal charges, and not ionic charges); a dative bond symbol is in terms of function, a sort of mini-mechanism used only in physical and inorganic chemistry), a dative bond symbol must be indicated from N to O (the donation of a dative bond explains the formal charges; because we're showing formal charges after dative bond formation, the dative bond is always from the +ve formal charged atom to the -ve formal charge atom; ie. the formal charges are a consequence of a dative bond).
The unpaired electron is indeed on N, which is why NO2 dimerizes to N2O4 (an equilibrium reaction), as well as why NO2 combines with NO to generate N2O3 (another equilibrium reaction).
Finally, H2 Chemistry students are required to state and explain the NO2 bond angle. Although the electron geometry is trigonal planar and the molecular geometry is v-shape or bent, but because half a lone pair occupies much less space or has much less repulsion, than either a lone pair or a bond pair, hence the bond angle is increased from 120 deg to 134 deg.
-
Thanks for the detailed answer, really brought across many things I have overlooked (e.g. octet concepts for Period 2)
I am still a bit unsure how you decided the nitrogen in the Case 1 has a formal charge of (+1).
I dug around online for a diagram and came across this

To me, formal charge on N = 5 - 2- 1/2(6) = 0.
Or have I done it wrongly?
-
Originally posted by atomos:
Thanks for the detailed answer, really brought across many things I have overlooked (e.g. octet concepts for Period 2)
I am still a bit unsure how you decided the nitrogen in the Case 1 has a formal charge of (+1).
I dug around online for a diagram and came across this

To me, formal charge on N = 5 - 2- 1/2(6) = 0.
Or have I done it wrongly?
The Kekule (many pple call it Lewis, but it should be called Kekule) structure in the image file you've given above is incorrect.In the correct structure, the N atom in NO2 has 3 bond pairs and 1 unpaired electron, which means it only has 4 valence electrons but it is in Group V, and thus has a unipositive formal charge. The singly bonded O atom has 3 lone pairs and 1 bond pair, which means it has 7 valence electrons but is in Group VI, and thus has a uninegative formal charge. Whenever you see positive and negative formal charges on adjacent atoms, you suspect a dative bond.
The following image shows the two resonance contributors and the resonance hybrid of NO2 :
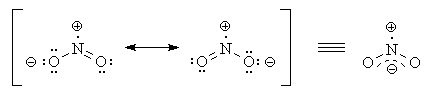
In the case of the nitrate ion (latin name) or nitrate(V) ion (stock name), there are two singly bonded O atoms with a uninegative formal charge. But there is only one dative bond, because the formal charge on the N atom is only unipositive. The other O atom's uninegative formal charge arose from the loss of a proton from its conjugate acid form HNO3, nitric acid (latin name) or nitric(V) acid (stock name).
The following are the three resonance contributors of the nitrate(V) ion.

The following depicts the resonance hybrid of the nitrate(V) ion.

The concept of resonance contributors and resonance hybrids, is essential for a proper understanding of Chemistry, and is something I insist my BedokFunland JC students understand. The vast majority of JC students up till the day they take their 'A' levels, still have a poor understanding of Chemistry, because most Singapore JCs do not teach fundamentally vital concepts such as formal charges and resonance, to 'A' level H2 Chemistry students, which is quite frankly an educational injustice.
-
Yup, I do agree with the need to cover resonance more properly.
I understood the correct structures of the NO2 and nitrate that you provided.
I guess my remaining concern is that if a student does come up with the structure that I posted in post 3.
Besides having prior knowledge to the conventionally accepted structure, what would be his reason to realise that it is "incorrect?" I mean, the formal charges on all atoms is still 0 (?) and octet is achieved for the atoms.
-
Originally posted by atomos:
Yup, I do agree with the need to cover resonance more properly.
I understood the correct structures of the NO2 and nitrate that you provided.
I guess my remaining concern is that if a student does come up with the structure that I posted in post 3.
Besides having prior knowledge to the conventionally accepted structure, what would be his reason to realise that it is "incorrect?" I mean, the formal charges on all atoms is still 0 (?) and octet is achieved for the atoms.

The reason why your structure (reproduced above) is incorrect, is because of oxidation states.
Oxidation State = Formal Charge + Electronegativity consideration.
(This is another one of my BedokFunland JC formulae, which Singapore JCs do not teach H2 Chemistry students, if for no other reason than the fact that many teachers themselves do not know this).
In your erroneous structure above, the OS of the O atom = Formal Charge + Electronegativity consideration = 0 + -1 = -1.
Because O atoms are more electronegative, they will bully their way through till they have an OS of -2 before they are satisfied. Thus the only time (in a stable species) when the OS of oxygen is not -2, is when the O atom is either bonded to another O atom (eg. peroxy groups), or when the O atom is bonded to (an even more electronegative) F atom.
Notice (in contrast) that in the correct structure of NO2 as I've described, the OS of both O atoms are -2.
Lastly, you mentioned "octet is achieved for the atoms" which is not true in your erroneous structure. Your singly bonded O atom has 2.5 lone pairs and 1 bond pair, which makes it 7 valence electrons in terms of stable octet (thus lacking a stable octet), and 6 valence electrons in terms of formal charge (thus having no formal charge, since O is in Grp VI).
-
Oh, you are right! For some reason, I kept viewing the singly bonded O as having 8 electrons when its not. That's a convicing reason to check other possible structures.
Regarding formal charges and electronegativity,
I had the idea that formal charges "assumes" covalent bonding and oxidation state "assumes" ionic bonding (while compounds do display a mixture of both).
What you did in your equation has made it more quantifiable.
I'm sure I'll be posting more queries as I plough though the other chapter in the guide books, but thanks for all your generous help in this. Really appreciate it.
-
Originally posted by atomos:
Oh, you are right! For some reason, I kept viewing the singly bonded O as having 8 electrons when its not. That's a convicing reason to check other possible structures.
Regarding formal charges and electronegativity,
I had the idea that formal charges "assumes" covalent bonding and oxidation state "assumes" ionic bonding (while compounds do display a mixture of both).
What you did in your equation has made it more quantifiable.
I'm sure I'll be posting more queries as I plough though the other chapter in the guide books, but thanks for all your generous help in this. Really appreciate it.
No problem, you're totally welcome. Keep up your good work with your students (teach them the missing concepts that their school teachers miss out), atomos!