Lurkers, register with SgForums and ask ur H2 Chem qns here!
-
Tys 2008 p3 q3a If the question ask state the reagents and conditions, is it necessary to write reagents:hcn conditions: trace amount of naoh,cold(10-20 degrees), or can we just write hcn with trace amount of naoh cold, without having to specifially write reagents: blabla conditions:blabla... and is trace amount of naoh considered as reagents or conditions? Ty
-
Originally posted by ArJoe:
Tys 2008 p3 q3a
If the question ask state the reagents and conditions, is it necessary to write reagents:hcn conditions: trace amount of naoh,cold(10-20 degrees), or can we just write hcn with trace amount of naoh cold, without having to specifially write reagents: blabla conditions:blabla…
and is trace amount of naoh considered as reagents or conditions? Ty
If P3, just combine. If P2, also can combine, unless the qn separated the reagents vs conditions into 2 different lines on the answer booklet. As for ur last qn, see CS Toh book "Carbonyl Compounds", Reaction with HCN. -
Thanks. For tys 2011 p3 q5d, for reagents for steps 1, i wrote sn and concentrated hcl, heat under reflux followed by addition of naoh(aq). Is it necessary to write addition of EXCESS naoh(aq). for step 2 i wrote naoh(aq). Need to write excess? And for step 2 the reaction is acid base neutralisation right? and for tys 2011 p3 q1e, for compound d the formula is c6h5conh2, is it necessary to draw all the bonds in conh2? Or can just draw benzene ring and just draw a bond to conh2 in position 1? Thanks for replying.
-
Originally posted by ArJoe:
Thanks. For tys 2011 p3 q5d, for reagents for steps 1, i wrote sn and concentrated hcl, heat under reflux followed by addition of naoh(aq). Is it necessary to write addition of EXCESS naoh(aq).
for step 2 i wrote naoh(aq). Need to write excess? And for step 2 the reaction is acid base neutralisation right?
and for tys 2011 p3 q1e, for compound d the formula is c6h5conh2, is it necessary to draw all the bonds in conh2? Or can just draw benzene ring and just draw a bond to conh2 in position 1? Thanks for replying.
ArJoe ah, from now on, you can start your own threads liao. Just name the thread "[H2 Chemistry] -" together with the name of the topic or some other relevant description.
In some cases, "excess" is required, in some other cases, it's optional. You should just strictly follow a reliable source (eg. CS Toh, or Chan Kim Seng, or George Chong, all these are better than most JCs' lecture notes), to play it safe.
For your specific context, check out these 3 (whichever you have) sources, you'll notice that they differ slightly, but the material from these 3 sources are all acceptable by Cambridge. So that indirectly answers your qn on whether "excess" is compulsory for this reaction. Look it up yourself.
Yes, it is.
You should always (even if the qn didn't specify full or displayed structural formula) show all bonds present in groups like CONH and CN to play safe. -
Hi UltimaOnline. I heard that there is a new syllabus for H2 Chemistry. How is it like? What are some of the changes?
-
Originally posted by Shafiq_6480:
Hi UltimaOnline. I heard that there is a new syllabus for H2 Chemistry. How is it like? What are some of the changes?
Update on 15 Jan 2016 : Oh yeah, 1 more little change in the new H2 Chem syllabus, Singapore A level students are now required to follow the new IUPAC Group numbers notation for the Periodic Table, instead of the A level simplified version of the CAS notation or old IUPAC notation. Eg. Halogens are now Group 17, not group VII. It's just a tiny insignificant change (nonetheless long overdue if you ask me), no worries.
Hardly any real changes for H2 Chem as far as the student is concerned (for MOE teachers, abandoning SPA is the real big change). Mostly just empty bureaucratic re-packaging of topics into "Core Ideas and Extension Topics", re-presentation of the "CURRICULUM FRAMEWORK", ie. how the topics are linked together thematically (something the H2 Bio syllabus already had all these years), plus some extra pedagogical bells & whistles (ie. more work for MOE teachers) called "Learning Experiences" and "Practices of Science" to (ostensibly) enhance the student learning experience and a deeper appreciation of the attitudes and ethical issues in the scientific approach. All of which are nothing to worry about (as far as the A level student is concerned).
Format wise, P1 now only has 30 MCQ instead of the existing 40 MCQ. P3 now has a Section B in which you choose 1 out of 2 qns (all Section A qns are compulsory), instead of the existing choose 4 out of 5 qns format. Planning is still 5% of the overall score, but has been shifted from P2 to P4 Practical Exam.
Which brings us to the biggest single change for the H2 Chem syllabus : SPA is being replaced by a one-time Paper 4 Practical Exam, due to ongoing problems with SPA since day 1 (which like so many ideas, sound good on paper but suffer from problems in practice), which I clearly foresaw when it was first implemented over a decade ago.
The syllabus content itself for H2 Chem is almost exactly the same, other than just adding and applying the thermodynamics Gibbs free energy formulae delta G = - n F Ecell (where F = Farady's constant 96485 coulombs) and delta G = - R T ln Kc (where R = Ideal gas constant 8.31446 m^3 Pa / K ), introducing the qualitative effect of the Nernst equation in electrochemistry, adding the Friedel-Crafts alkylation and acylation reaction conditions and mechanisms, adding the ortho-para vs meta directing nature of benzene ring substituents (ie. identifying electron-donating/withdrawing by induction vs resonance, and how these stabilize or destabilize the resonance hybrids of the different positional isomers of the tetrahedral sp3 intermediate of benzene during electrophilic aromatic substitution), plus a couple more little additions in some other topics.
None of which I haven't *already* been teaching my BedokFunland JC students all these years (and much more, so as to enable my students to more correctly understand, not just memorize, Chemistry, ie. giving my students the H3 Chem and Olympiad Chem advantage over H2 Chem students). -
Hi UltimaOnline! Thank you for the detailed breakdown! Did any of the contents from the previous syllabus was taken out for the new syllabus? Was thinking of getting the notes from my seniors.
-
Originally posted by Shafiq_6480:
Hi UltimaOnline! Thank you for the detailed breakdown! Did any of the contents from the previous syllabus was taken out for the new syllabus? Was thinking of getting the notes from my seniors.
No, nothing was taken out, only a bit more stuff added in. -
Racoon : "Candy floss for me? Wow thank you so much! Just lemme wash it first..."
BedokFunland JC : "O hydrogen bonding, thou art heartless... O positive entropy change, thou art cruel..."was it that this happened , that the candyfloss, which is made up of water soluble compounds (99% sugar, +coloring) , dissolve in water, due to the H-bond formed between water molecules and sugar molecules, which is a process of positive entropy change?
-
Originally posted by Flying grenade:
Racoon : "Candy floss for me? Wow thank you so much! Just lemme wash it first..."
BedokFunland JC : "O hydrogen bonding, thou art heartless... O positive entropy change, thou art cruel..."was it that this happened , that the candyfloss, which is made up of water soluble compounds (99% sugar, +coloring) , dissolve in water, due to the H-bond formed between water molecules and sugar molecules, which is a process of positive entropy change?
Yes, of course ;Þ -
Ultima,
Is it :
the new, modern, IUPAC notation for the groups elements of the periodic table, is labelled as 1, 2, 3, .. ,18 correct? the greek notation I, II, III has long been obsolete, is it? in fact, that isn't even true in the first place? there was like e.g. VIIB type of naming.
https://en.wikipedia.org/wiki/Group_(periodic_table)
-
Originally posted by Flying grenade:
Ultima,
Is it :
the new, modern, IUPAC notation for the groups elements of the periodic table, is labelled as 1, 2, 3, .. ,18 correct? the greek notation I, II, III has long been obsolete, is it? in fact, that isn't even true in the first place? there was like e.g. VIIB type of naming.
https://en.wikipedia.org/wiki/Group_(periodic_table)
Both the new IUPAC and the CAS notation are concurrently being used worldwide. The new Singapore H2 syllabus will follow the new IUPAC notation, ie. Halogens are Group 17. -
what's cas?
http://www.seab.gov.sg/content/syllabus/alevel/2016Syllabus/9647_2016.pdf
didn't notice any change in this )):
did you know about the other subjects, whether is there change in syllabus?
-
Originally posted by Flying grenade:
what's cas?
http://www.seab.gov.sg/content/syllabus/alevel/2016Syllabus/9647_2016.pdf
didn't notice any change in this )):
did you know about the other subjects, whether is there change in syllabus?
https://www.cas.org/
The syllabus is officially examined in 2017, which means 2016 JC1 students start using the new syllabus right now.
For H2 Physics, ask Eagle. For H2 Math, ask Eagle and/or WeeWS. Make a new thread so they'll see it (as they may not look inside a Chem thread). -
ok cool, understood. and also saw ur breakdown of the new syllabus above.
thanks!
-
Hi ultimaonline, according to my teacher, most people will get full 15% for spa or at least close to that like 13 to 14% in all jcs, so bell curve for h2 chem to get A is around 80% because of spa. Since i was thinking schools will definitely prepare their students very adequately for spa, so is it true that the a grade will be around 80% due to most students getting full marks for spa?
-
Originally posted by ArJoe:
Hi ultimaonline, according to my teacher, most people will get full 15% for spa or at least close to that like 13 to 14% in all jcs, so bell curve for h2 chem to get A is around 80% because of spa. Since i was thinking schools will definitely prepare their students very adequately for spa, so is it true that the a grade will be around 80% due to most students getting full marks for spa?
That's another prob with SPA, which is why it's being done away with. Inconsistent marking across schools. Anyway, no worries. A grade is almost always around 75%, not 80%. -
what are the reason behind the group numbers??
tried to research but to no availfrom bbc bitesize
https://www.dropbox.com/s/mtfhzok1d1jmh46/Capture.PNG?dl=0
: Elements in the same group in the periodic table ... their atoms have the same number of electrons in the highest occupied energy level.from bmat specimen paper
https://www.dropbox.com/s/0nihqt0kd17dzqt/Capture2.PNG?dl=0
: the number of the group shows the number of electrons in the outer shell of the atom;how are these true??
e.g. Neon :1s2 2s2 2p6 3s2, 3p6
number of e- in highest occupied energy level = 6 , number of e- in the outer shell of atom= 8 ,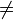 18 ??
18 ?? -
Originally posted by Flying grenade:
what are the reason behind the group numbers??
tried to research but to no availfrom bbc bitesize
https://www.dropbox.com/s/mtfhzok1d1jmh46/Capture.PNG?dl=0
: Elements in the same group in the periodic table ... their atoms have the same number of electrons in the highest occupied energy level.from bmat specimen paper
https://www.dropbox.com/s/0nihqt0kd17dzqt/Capture2.PNG?dl=0
: the number of the group shows the number of electrons in the outer shell of the atom;how are these true??
e.g. Neon :1s2 2s2 2p6 3s2, 3p6
number of e- in highest occupied energy level = 6 , number of e- in the outer shell of atom= 8 ,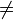 18
??
18
??
This is true specifically for d block elements, including transition metals. Eg. Cu is Group 11, since it has 4s1 3d10 electron configuration, ie. 1+10 = 11 valence electrons. For d block elements, the 3d subshell is considered part of the valence shell, since 3d electrons participate in bonding. -
http://papers.gceguide.com/A%20Levels/Chemistry%20(9701)/9701_w15_qp_42.pdf
http://papers.gceguide.com/A%20Levels/Chemistry%20(9701)/9701_w15_ms_42.pdf
Please take a look at Q3 part b(ii).
I dont understand how nitrate ion can act as ligand while BF3 cannot. In both NO3- and BF3, central atom(N and B respectively) have no lone pair of electrons which means by this logic, both NO3- and BF3 cannot act as ligands.
By another logic, the O-atoms in NO3- have lone pairs to donate and so do the F-atoms in BF3 so both should be able to function as ligands.
Why does NO3- act as ligand while not BF3?
Thank you for explaining this.
-
Originally posted by Light5:
http://papers.gceguide.com/A%20Levels/Chemistry%20(9701)/9701_w15_qp_42.pdf
http://papers.gceguide.com/A%20Levels/Chemistry%20(9701)/9701_w15_ms_42.pdf
Please take a look at Q3 part b(ii).
I dont understand how nitrate ion can act as ligand while BF3 cannot. In both NO3- and BF3, central atom(N and B respectively) have no lone pair of electrons which means by this logic, both NO3- and BF3 cannot act as ligands.
By another logic, the O-atoms in NO3- have lone pairs to donate and so do the F-atoms in BF3 so both should be able to function as ligands.
Why does NO3- act as ligand while not BF3?
Thank you for explaining this.
NO3- is anionic, electron-rich, and hence can function as a nucleophile, Bronsted-Lowry base, ligand, and Lewis base. The donor atom (donating the dative covalent bond) would be any of the O atoms with a uninegative formal charge (in the resonance hybrid, all 3 O atoms bear a 2/3 negative formal charge and are thus equivalent).
BF3 is a neutral molecule, but because the B atom lacks a stable octet, it is in that sense electron-deficient (even though the B atom is formally neutral without any formal charge, but it does bear a significant partial positive charge, due to the electron-withdrawing by induction effects of the electronegative F atoms, though the lack of a stable octet is the most important consideration for its Lewis acidic behaviour), and thus BF3 can function as an Lewis acid (with the electrophilic B atom as the recipient atom accepting the dative covalent bond).
F being the most electronegative element, has low propensity for donating dative bonds (due to the unipositive formal charge it would consequently incur). Which is why BF3 is certainly a lot more Lewis acidic (or electrophilic) than it is Lewis basic (or nucleophilic). Rather, it is the F- ion (ie. fluoride ion, with a uninegative formal & ionic charge), being electron-rich, that does indeed function as a strong Lewis base, Bronsted-Lowry base, nucleophile and ligand, with a strong (due to effective overlap of the F atom's 2nd electron shell orbitals) dative covalent bond (with significant ionic character) thusly formed. -
http://papers.gceguide.com/A%20Levels/Chemistry%20(9701)/9701_w15_qp_42.pdf
http://papers.gceguide.com/A%20Levels/Chemistry%20(9701)/9701_w15_ms_42.pdf
In Q3(c) why isnt MnO4- formed when Mn+2 reacts with H2O2 despite the fact that Eo of H2O2/H2O is +1.77 and that for MnO4/Mn+2 is lower so theoretically this reaction is possible...so why is Mn+3 formed?
In Q7 part b(ii) why is an ionic compound i.e NH2+Br- formed with CH3CH2Br and why isnt HBr formed along with -NHCH2CH3 if the reaction is nucleophilic substitution?
http://papers.gceguide.com/A%20Levels/Chemistry%20(9701)/9701_w15_qp_52.pdf
http://papers.gceguide.com/A%20Levels/Chemistry%20(9701)/9701_w15_ms_52.pdf
In Q1(a) why cant volume/24000 method be used..and isnt PV=nRT defined only for ideal gases??
In Q1(c) if we increase pressure, then volume reduces so assuming we use Mr=mRT/PV (as is used by examiner)..shouldnt PV remain constant meaning Mr value is unaffected...i understand how in Q1(b) the calculated value of Mr will be lower than actual value but even if we increase pressure to reduce volume to try to compensate for the incorrectly recorded temperature, shouldnt PV remain constant and so no effect on calculated value of Mr from (b)...please explain why my logic is wrong?
Thank You
-
Originally posted by Light5:
http://papers.gceguide.com/A%20Levels/Chemistry%20(9701)/9701_w15_qp_42.pdf
http://papers.gceguide.com/A%20Levels/Chemistry%20(9701)/9701_w15_ms_42.pdf
In Q3(c) why isnt MnO4- formed when Mn+2 reacts with H2O2 despite the fact that Eo of H2O2/H2O is +1.77 and that for MnO4/Mn+2 is lower so theoretically this reaction is possible...so why is Mn+3 formed?
In Q7 part b(ii) why is an ionic compound i.e NH2+Br- formed with CH3CH2Br and why isnt HBr formed along with -NHCH2CH3 if the reaction is nucleophilic substitution?
http://papers.gceguide.com/A%20Levels/Chemistry%20(9701)/9701_w15_qp_52.pdf
http://papers.gceguide.com/A%20Levels/Chemistry%20(9701)/9701_w15_ms_52.pdf
In Q1(a) why cant volume/24000 method be used..and isnt PV=nRT defined only for ideal gases??
In Q1(c) if we increase pressure, then volume reduces so assuming we use Mr=mRT/PV (as is used by examiner)..shouldnt PV remain constant meaning Mr value is unaffected...i understand how in Q1(b) the calculated value of Mr will be lower than actual value but even if we increase pressure to reduce volume to try to compensate for the incorrectly recorded temperature, shouldnt PV remain constant and so no effect on calculated value of Mr from (b)...please explain why my logic is wrong?
Thank You
Q3c) This Cambridge question is problematic, because actually you do *not* get Mn3+(aq) either. Any MnO4-(aq) generated from the oxidation of Mn2+ by H2O2, being a strong oxidizing agent, will immediately react with H2O2 to be reduced back to Mn2+ and/or MnO2 (depending on pH), so you don't get MnO4-(aq) as a stable product. But because Mn3+ is also *extremely* unstable and a strong oxidizing agent, any Mn3+(aq) generated from the oxidation of Mn2+ by H2O2, will also immediately react with H2O2 to be reduced back to Mn2+, and/or undergo hydrolysis and/or precipitate out (depending on pH) to any of the following significantly more thermodynamically and kinetically stable species : pentaaqua-monohydroxo-manganese(III) complex ion [Mn(OH)(H2O)5]2+(aq), and/or manganese(III) oxide-hydroxide MnO(OH)(s), and/or manganese(III) oxide Mn2O3(s), and/or manganese(II,III) oxide MnO.Mn2O3(s), and/or undergo disproportionation into Mn2+(aq) and MnO2(s).
So this Cambridge question is problematic. If MnO4- is rejected as an answer, then Mn3+ should also be rejected. If Mn3+ is accepted (based simply on redox potentials), then MnO4- should also be accepted. Don't worry too much about this (every year in every Cambridge Chemistry paper, there's bound to be a couple of problematic questions, but since all candidates will struggle with such questions together, and since grades are assigned on a bell-curve anyway, so it's no real cause for worry).
In Q7 part b(ii). The immediate product of an SN2 reaction (draw the mechanism and you'll see why) is actually the ionic salt (ie. ammonium halide), which exists in equilibrium with the Bronsted-Lowry proton-transferred species of amine + hydrogen halide (ie. the products you suggested). But since the question specified "the products are more soluble in water than in organic solvents", the question is testing if you understand that this refers to ionic species rather than molecular species (as stronger ion-permanent dipole interactions ensure aqueous solubility) and hence Cambridge will only accept the ionic salt as the required answer for this question.
As an aside, HCl (and any hydrogen halide HX) could also undergo electrophilic addition with alkenes (though this reaction is more controlled in the gaseous state, because in the aqueous state water is a competing nucleophile), but since this question Cambridge specified the molecular formula of the desired product, hence the required answer does not involve this side reaction.
Q1a). First of all, 24dm3 is a terribly inaccurate approximation itself obtained from PV=nRT (if you carry out the calculations, you'll find 24dm3 to be a 2 sig fig approximation, not even acceptable for 3 sig fig, so only use 24dm3 when the Cambridge question specifies you can use it), so your 24dm3 is even less accurate than using PV=nRT (to at least 3 sig fig). And yes, it's for ideal gases, which at A levels you have no choice but to use the assumption that the ideal gas formula is an acceptable approximation for the properties of the real gas (otherwise if you want greater accuracy, you'll have to use the Van der Waals equation, taught only at Uni level).
The second and more relevant reason for this question, is that at room temperature and pressure, this species exists as a liquid (it only exists as a gas when you heat it beyond room temperature), which means that under the conditions this species exists as a gas (ie. *not* at rtp), you cannot use 24dm3 gaseous molar volume (which is a 2 sig fig approximation for rtp *only*), and hence you *must* use PV=nRT.
Q1b(i&ii). LOL this is where "2 wrongs make a right". You would be right if you increased the pressure *and* you honestly and correctly used the newly increased pressure in your calculations. But Cambridge (evidently from their mark scheme) is implying that you are to cunningly & stealthily increase the pressure *but* you continue to dishonestly use the old lower pressure value in your calculations, ie. using a wrong pressure value to rectify a wrong temperature value, to obtain the (more) correct molar mass of the gas. -
How are free radicals electrically neutral??
-
Originally posted by Flying grenade:
How are free radicals electrically neutral??
It's about formal charges. Consider the methyl radical. The C atom has 3 bond pairs and 1/2 a lone pair. That's 7 valence electrons in terms of a stable octet (ie. lacks a stable octet), and 4 valence electrons in terms of formal charge, and because C is in Group 14, hence the C atom has no formal charge, and hence the methyl radical is a neutral molecule without any ionic charge.