Lurkers, register with SgForums and ask ur H2 Chem qns here!
-
Hi Ultima, may i ask
What are the factors affecting decomposition?
From wiki :https://en.m.wikipedia.org/wiki/Hydrogen_peroxide The rate of decomposition increases with rising temperature, concentration and pH, with cool, dilute, acidic solutions showing the best stability.
I dont understand why acidic solutions would render h2o2 more stable
-
Originally posted by Flying grenade:
Hi Ultima, may i ask
What are the factors affecting decomposition?
From wiki :https://en.m.wikipedia.org/wiki/Hydrogen_peroxide The rate of decomposition increases with rising temperature, concentration and pH, with cool, dilute, acidic solutions showing the best stability.
I dont understand why acidic solutions would render h2o2 more stable
Eh? You haven't enlist yet meh? Are you retaking your A levels during NS in 2016 or 2017? Are you sure your OC and CO will grant you leave during the A level exam period? As they love to remind you in the SAF, this is a privilege, not an entitlement.There are many mechanism pathways for H2O2 decomposition, depending on whether or not, and exactly which, catalysts are involved. Since experimental evidence has indicated the rate of H2O2 decomposition (with no catalyst) increases with pH, you can therefore deduce that (this particular) decomposition mechanism is inhibited by H+, or involves OH-. If you like, you can try drawing out the various possible mechanisms, and see which mechanisms are consistent with the experimental data (eg. how pH affects rate of decomposition, order of reaction wrt H2O2, etc), and hence possible.
-
Are there general factors that always affects decomposition? Is it safe to say that higher temperature and presence of catalyst is sure to increase the rate of decomposition?
-
Originally posted by Flying grenade:
Are there general factors that always affects decomposition? Is it safe to say that higher temperature and presence of catalyst is sure to increase the rate of decomposition?
By definition of catalyst, yes. Higher temperature usually increases both the rate and position of equilibrium towards decomposition in most cases, but there are some cases in which the decomposition is exothermic, which means lowering temperature shifts the position of equilibrium towards decomposition instead of increasing temperature. However, since increasing temperature increases both kf and kb, hence both the forward and backward reactions are still increased, even if the position of equilibrium shifts away from decomposition. -
Hi sir Ultima, may i ask,
a Lewis diagram = Dot and Dot structure
a Kekule diagram = Line and Dot structure
a Dot and Cross diagram = Dot and Cross structure
Many people, including teachers in Singapore JCs, are confused about the difference between these terms, and often use the term "Lewis structure" when they mean "Kekule structure"; Cambridge uses the term "Displayed Structural Formula".
For 'A' level purposes, Cambridge will accept any of the above, or even hybrid variants between.
a Lewis diagram = Dot and Dot structure
What dyou mean by Dot and Dot?
Thank you !
-
Originally posted by Flying grenade:
Hi sir Ultima, may i ask,
a Lewis diagram = Dot and Dot structure
a Kekule diagram = Line and Dot structure
a Dot and Cross diagram = Dot and Cross structure
Many people, including teachers in Singapore JCs, are confused about the difference between these terms, and often use the term "Lewis structure" when they mean "Kekule structure"; Cambridge uses the term "Displayed Structural Formula".
For 'A' level purposes, Cambridge will accept any of the above, or even hybrid variants between.
a Lewis diagram = Dot and Dot structure
What dyou mean by Dot and Dot?
Thank you !
Lewis structures are dot-dot diagrams :
https://www.nyu.edu/classes/tuckerman/adv.chem/lectures/lecture_11/node1.htmlKekule structures are bond-line diagrams :

http://chemwiki.ucdavis.edu/Core/Organic_Chemistry/Case_Studies/What_is_Organic%3FSingapore JC teachers often wrongly call Kekule structures as Lewis structures. But it doesn't really matter, coz Cambridge doesn't use either term, rather, Cambridge will call it "displayed structural formula" or "full structural formula" (ie. the Kekule structure, with lone pairs not required to be shown unless the molecular or ionic geometry is also required), as opposed to dot-&-cross structures.
Lastly, Singapore JC teachers also wrongly call the resonance contributor of benzene as "the Kekule structure of benzene". (Actually here, it's not totally the Singapore JC teachers' error, becoz in a way it was Kekule's own error when he drew out the structure of benzene over a hundred years ago, but don't blame him, he was still the first human to come closest to the correct structure of benzene (you should check out the alternative proposed structures of benzene by other rival chemists), and back then, humans didn't understand the concept of resonance contributors and resonance hybrids (which Singapore JCs only properly teach to H3 Chem & OIympiad Chem students, depriving poor H2 Chem students of a proper understanding of Chemistry), so Mr Kekule was still the best man with the best idea back then).
This (what Singapore JCs call as the "Kekule structure of benzene") is more correctly called the Kekule/Lewis structure and skeletal structural formula (respectively) for the resonance contributor of benzene :
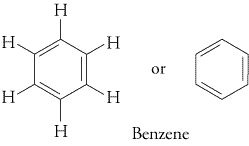
http://preparatorychemistry.com/Bishop_Resonance.htmThis (what Singapore JCs call as the "correct structure of benzene") is more correctly called the Kekule/Lewis structure and skeletal structural formula for the resonance hybrid of benzene :

http://www.tutorvista.com/chemistry/physical-properties-of-benzene
http://chemistrywithelevens.blogspot.sg/2012/05/rings-and-cyclones.htmlLastly, note that Cambridge will accept the skeletal formula for benzene even if the question specifies "full or displayed structural formula". And when drawing structures or mechanisms, Cambridge will accept either the resonance hybrid (ie. with the circle in the middle, which Singapore JCs anally insist you draw this instead of the resonance contributor with alternating double and single bonds, because they're afraid you JC H2 students are not intelligent enough to understand the intricacies of the differences between resonance contributor and resonance hybrid), or the resonance contributors (ie. with the alternating double and single bonds, which is actually the more correct structure to use when drawing reaction mechanisms, as you'll learn in the University, if you take Chemistry or Medicine in the University).
Cambridge will accept either the resonance contributor or hybrid, so no worries. Of course, the A level shortcut of using the resonance hybrid saves time, so go ahead and use it (most students can't even complete doing all questions in the A level paper during the A level exams). But if you want a deeper understanding of Chemistry, and also for some of the more challenging exam A grade distinction questions (eg. one of my BedokFunland JC H2 Chemistry questions : Under certain conditions, para- aminophenol, also known as para- hydroxyphenylamine, can be oxidized to generate a product with molecular formula C6H5NO. By drawing the simplified free radical mechanism to represent the reaction, elucidate the structure of the product.), you'll find that using the resonance contributor is actually superior to using the resonance hybrid.
-
Why the requirement for new users to post 20 replies on any thread, with 5 minutes waiting interval, only then able to post a thread on the forums?
-
Test
-
Woah godly chemistry replies , thank you
-
ACJC/2014/P3/QN1(a)
I made a careless mistake doing this qn, i was required to draw dot and cross for N2O4, but i carelessly drew N2O2
However i realised that the dot and cross was good to draw with, in the sense all atoms in this molecule got full octet
https://www.dropbox.com/s/0dtmulqzsnxdj24/20160318_010332.jpg?dl=0
(Update, i just realised that drawing suits for Hydrazine, N2H4, maybe thats why N2O2 do not exist?)
However I'm surprised N2O2 doesn't exist in nature(or does it) !?
https://en.m.wikipedia.org/wiki/Nitrogen_oxide
No N2O2 in this wiki page
https://www.dropbox.com/s/o3hnr80cqvm29ah/Screenshot_2016-03-18-01-03-49.png?dl=0
Why doesnt N2O2 exist?
-
Originally posted by Flying grenade:
ACJC/2014/P3/QN1(a)
I made a careless mistake doing this qn, i was required to draw dot and cross for N2O4, but i carelessly drew N2O2
However i realised that the dot and cross was good to draw with, in the sense all atoms in this molecule got full octet
https://www.dropbox.com/s/0dtmulqzsnxdj24/20160318_010332.jpg?dl=0
(Update, i just realised that drawing suits for Hydrazine, N2H4, maybe thats why N2O2 do not exist?)
However I'm surprised N2O2 doesn't exist in nature(or does it) !?
https://en.m.wikipedia.org/wiki/Nitrogen_oxide
No N2O2 in this wiki page
https://www.dropbox.com/s/o3hnr80cqvm29ah/Screenshot_2016-03-18-01-03-49.png?dl=0
Why doesnt N2O2 exist?
N2O2 exists, but under standard conditions at 25 deg C, it decomposes into NO monomers with thermodynamically favorable positive entropy change, with the position of equilibrium lying so far towards the NO monomer side that the molarity of N2O2 dimer is negligible. You need to cool NO into its liquid state (−152 °C) to have a significant molarity of N2O2 dimer. -
AJC/2014/P3/Q1 (b)
Qn : BeCl2 has properties similar to those of aluminium chloride, AlCl3. Write a balanced equation to show the reaction of beryllium chloride with limited amount of water.
Answer : BeCl2 + H2O -> BeO + HCl
Or BeCl2 + 2H2O -> Be(OH)2 + 2HCl
But
AlCl3 + 6H2O -> [Al(H2O)6]3+ + 3Cl-
[Al(H2O)5(OH)]2+ 《》[Al(H2O)5(OH)]2+ +H+
How to solve this qn/ train of thought?
First thing first is it detect the phrase 'limited water'?
-
Originally posted by Flying grenade:
ACJC/2014/P3/Q1 (b)
Qn : BeCl2 has properties similar to those of aluminium chloride, AlCl3. Write a balanced equation to show the reaction of beryllium chloride with limited amount of water.
Answer : BeCl2 + H2O -> BeO + HCl
Or BeCl2 + 2H2O -> Be(OH)2 + 2HCl
But
AlCl3 + 6H2O -> [Al(H2O)6]3+ + 3Cl-
[Al(H2O)5(OH)]2+ 《》[Al(H2O)5(OH)]2+ +H+
How to solve this qn/ train of thought?
First thing first is it detect the phrase 'limited water'?
It's not ACJC/2014/P3/Q1(b). Check and give me the correct question data. -
Originally posted by UltimaOnline:
It's not ACJC/2014/P3/Q1(b). Check and give me the correct question data.I am sorry.
AJC/2014/P3/QN1 (b).
-
Originally posted by UltimaOnline:
N2O2 exists, but under standard conditions at 25 deg C, it decomposes into NO monomers with thermodynamically favorable positive entropy change, with the position of equilibrium lying so far towards the NO monomer side that the molarity of N2O2 dimer is negligible. You need to cool NO into its liquid state (−152 °C) to have a significant molarity of N2O2 dimer.I was under the impression that dimer can only be formed by a dative bond, from one lone pair of an atom to the empty orbital of another atom.
Doesnt seem like N2O2 has a dative bond
Can help further elucidate /check my drawing above?
Thank you Ultima
-
Originally posted by Flying grenade:
I am sorry.
AJC/2014/P3/QN1 (b).
Yah, it's becoz of limited water, so hydrolysis of BeCl2(s) goes only so far as to generate BeO(s) or Be(OH)2(s), Cambridge will accept either equation, unless otherwise specified by the question. To get acidic solution like AlCl3(aq) or MgCl2(aq) via proton dissociation from water ligands, you need excess water :[Be(H2O)4]2+(aq) + 2Cl-(aq) + H2O(l) --> [Be(OH)(H2O)3]+(aq) + 2Cl-(aq) + H3O+(aq)
-
Originally posted by Flying grenade:
I was under the impression that dimer can only be formed by a dative bond, from one lone pair of an atom to the empty orbital of another atom.
Doesnt seem like N2O2 has a dative bond
Can help further elucidate /check my drawing above?
Thank you Ultima
Formation of dimers needn't necessarily involve dative covalent bonds lah, depending on the exact species involved, dimers can formed via hydrogen bonds or normal (ie. non-dative) covalent bond, especially for free radicals (they dimerize in a free-radical termination step, while dissociation into monomers is a free-radical initiation step).Ur drawing of N2O2 is fine, now draw the electron-flow curved-arrow reaction-mechanism for both the forward (free-radical termination) reaction to generate the N2O2 dimer, as well as the backward (free-radical initiation) reaction to dissociate the N2O2 dimer into NO monomers.
A very similar question was asked by Cambridge a couple of years ago, but many students couldn't do it, coz many Singapore JCs didn't teach their students how to properly draw the curved arrow mechanism for termination steps (most schools just teach their students to write the mechanism, not draw it with curved arrows).
-
Can give example of H-bond dimers??
Can give examples of (normal) covalent bonded dimers? N2O4?
-
Originally posted by Flying grenade:
Can give example of H-bond dimers??
Can give examples of (normal) covalent bonded dimers? N2O4?
Yah, N2O4 is a dimer of NO2, and NO2 is a monomer of N2O4. The terms monomer and dimer are relationship adjectives, and have no meaning on its own. So "N2O4 is a dimer" is a wrong statement, but "N2O4 is a dimer of NO2 monomers" is a correct statement.For the most commonly asked at A levels H-bond dimer, click on the link below, followed by clicking on "images".
-
Thank you!
Dative covalent bonded dimers are Al2Cl6? And Al2Cl6 are dimers of AlCl3 monomers?
-
Originally posted by Flying grenade:
Thank you!
Dative covalent bonded dimers are Al2Cl6? And Al2Cl6 are dimers of AlCl3 monomers?
Yes of course. -
Why NaF has higher mp than CsCl?
CsCl has greater covalent character (hence lower mp), while NaF has greater ionic character (hence higher mp). When two elements have greater electronegativity difference (eg. Na vs F), there will be greater ionic character. When two elements have smaller electronegativity difference (eg. Cs vs Cl), there will be greater covalent character.
Do we need to explain why greater ionic character means higher mp than covalent character ones,
Or why ionic bonds are stronger than covalent bonds?
-
Having said that, there is a significant difference between CO2 and XeF2, which is : in CO2, the central partially positively charged C atom has no lone pairs, while the central partially positively charged Xe atom has 3 lone pairs. Therefore the (relative) magnitude of permanent dipole - permanent dipole attractions will be expected to be more significant for CO2 than for XeF2. And yet XeF2 has a much higher melting point than CO2, because XeF2 has a lot more electrons, has much larger molecular size, and therefore has significantly more polarizable electron charge clouds.)
Why CO2's Pd-Pd attraction is relatively stronger than XeF2's Pd-Pd attraction??
I thought XeF2 would have a larger net dipole moment than CO2, hence more significant Pd-Pd than CO2??
-
Btw CO2 has no net dipole moment, right?
-
Originally posted by Flying grenade:
Why NaF has higher mp than CsCl?
CsCl has greater covalent character (hence lower mp), while NaF has greater ionic character (hence higher mp). When two elements have greater electronegativity difference (eg. Na vs F), there will be greater ionic character. When two elements have smaller electronegativity difference (eg. Cs vs Cl), there will be greater covalent character.
Do we need to explain why greater ionic character means higher mp than covalent character ones,
Or why ionic bonds are stronger than covalent bonds?
No need to explain (because depending on exact species, sometimes covalent character increases melting point, sometimes it decreases melting point, for reasons far beyond A levels), for A level purposes, just state "significant covalent character" or "significant ionic character" as a reason for the deviation. Some ionic bonds are stronger than some covalent bonds, and some covalent bonds are stronger than some ionic bonds. Chemistry is a microcosm of real-life, which is wonderfully, beautifully, infinitely complex.