Lurkers, register with SgForums and ask ur H2 Chem qns here!
-
Originally posted by Flying grenade:
Ultima, everytime u take the A levels, your paper 1 - 5 all 100% correct?
Buddha said, "If I don't go to Hell, who will go to Hell?"BedokFunland JC said, "If I don't get A grade, who will get A grade?"
Because I know how Cambridge marks, I know the distinction A grade boundary and I know how the bell-curve works, hence I can choose to skip whichever questions or practical procedures I'm not interested in doing, and still get my A grade every year. So I don't get 100% full marks because I deliberately skip many questions or practical procedures, but the questions that I choose to do will be 99.9% correct, give or take a couple of silly careless mistakes (you don't actually need to outrun the bear, you only need to outrun the other guy who is also being chased by the bear).
-
Why Buddha said that? Isn't it supposed to be like if Buddha don't go to heaven, who will? Idk, havent heard that aphorism before. Or does the sentence hold some benevolent meaning?
Ultima, how fast do you complete paper 1 - 5? Like, 30 min in advance? Ur time management very good?
Do u check ur paper?
Do u sleep in the exam hall?
U always go vj/tj take the exam?
Do u check for the correct number of printed pages?
Do u highlight /annotate / underline/circle /tick on the question paper?
Do u have any pre exam or during exam good habits?
Do u use gc?
Do u eat healthily and exercise regularly?
-
Originally posted by Flying grenade:
Why Buddha said that? Isn't it supposed to be like if Buddha don't go to heaven, who will? Idk, havent heard that aphorism before. Or does the sentence hold some benevolent meaning?
Ultima, how fast do you complete paper 1 - 5? Like, 30 min in advance? Ur time management very good?
Do u check ur paper?
Do u sleep in the exam hall?
U always go vj/tj take the exam?
Do u check for the correct number of printed pages?
Do u highlight /annotate / underline/circle /tick on the question paper?
Do u have any pre exam or during exam good habits?
Do u use gc?
Do u eat healthily and exercise regularly?
Eh join my tuition then we talk about all these lah. Hurry up leh, less than 7 months to A levels, you wanna wait till 1 week before A levels than join izzit? Like that even Buddha also face-palm, cannot help you also.
-
Both molecules contain polar chlorine-carbon bonds, but in the cis isomer they are both on the same side of the molecule. That means that one side of the molecule will have a slight negative charge while the other is slightly positive. The molecule is therefore polar.
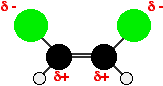
Because of this, there will be dipole-dipole interactions as well as dispersion forces - needing extra energy to break.
Is it cis isomers have keesom pdpd IMFs + london dispersion IDID IMFs,
Trans only have ID-ID IMFs?
From http://www.chemguide.co.uk/basicorg/isomerism/geometric.html
-
Originally posted by Flying grenade:
Both molecules contain polar chlorine-carbon bonds, but in the cis isomer they are both on the same side of the molecule. That means that one side of the molecule will have a slight negative charge while the other is slightly positive. The molecule is therefore polar.

Because of this, there will be dipole-dipole interactions as well as dispersion forces - needing extra energy to break.
Is it cis isomers have keesom pdpd IMFs + london dispersion IDID IMFs,
Trans only have ID-ID IMFs?
From http://www.chemguide.co.uk/basicorg/isomerism/geometric.html
Yes. -
Do we need to include PdId debye IMFs?
-
Originally posted by UltimaOnline:
Yes.Thanks godzilla and buddha fusion
-
Alkyl groups like methyl groups tend to "push" electrons away from themselves. You again get a polar molecule, although with a reversed polarity from the first example.
Can write colloquial terms in quotations e.g."push" electrons away from themselves, or use quotations marks in exams??
-
Originally posted by Flying grenade:
Do we need to include PdId debye IMFs?
No need. Include Debye forces only when it's the most relevant force. -
Originally posted by Flying grenade:
Alkyl groups like methyl groups tend to "push" electrons away from themselves. You again get a polar molecule, although with a reversed polarity from the first example.
Can write colloquial terms in quotations e.g."push" electrons away from themselves, or use quotations marks in exams??
Use quotation marks when using colloquial terms, and use colloquial terms only in addition to proper keywords, or when there's no choice (eg. you forgot the proper keyword), or when explaining or elaborating a concept that you already gave the keyword for. -
Hi Ultima, may i ask you,
I know the priority of the functional groups for aliphatic molecules as shown here :
https://www.dropbox.com/s/25ncr8p5i5y1e9x/20150107_153638-1.jpg?dl=0
However may i ask about the priority of the functional groups for aromatic molecules?
I was confused about this :
https://en.m.wikipedia.org/wiki/2-Nitrotoluene
Its called 2nitromethylbenzene here, i.e. toluene has higher priority?
Compared to https://en.m.wikipedia.org/wiki/Chlorotoluene, 1-chloro-2-methylbenzene , i.e. chlorine has higher priority here?
-
Originally posted by Flying grenade:
Hi Ultima, may i ask you,
I know the priority of the functional groups for aliphatic molecules as shown here :
https://www.dropbox.com/s/25ncr8p5i5y1e9x/20150107_153638-1.jpg?dl=0
However may i ask about the priority of the functional groups for aromatic molecules?
I was confused about this :
https://en.m.wikipedia.org/wiki/2-Nitrotoluene
Its called 2nitromethylbenzene here, i.e. toluene has higher priority?
Compared to https://en.m.wikipedia.org/wiki/Chlorotoluene, 1-chloro-2-methylbenzene , i.e. chlorine has higher priority here?
As far as professional chemists are concerned, so-called priorities of functional groups in naming organic molecules (in contrast, Cahn–Ingold–Prelog priority rules are clear, logical and must be consistently followed for uniformity and unambiguity's sake) are merely IUPAC guidelines (not strict rules), what's far more important (and critical) is that the species is unambiguously identified. Which means there are multiple correct or acceptable names for most organic molecules. For instance, look at the "Other names" below the "IUPAC name" for 2-Nitrotoluene here : https://en.wikipedia.org/wiki/2-Nitrotoluene. All these "other names" are also correct, IUPAC is a just a guideline.For A level purposes, Cambridge too, allows for flexibility in naming as long as it is unambiguous. And on a more relevant and practical note for JC students, is that Cambridge doesn't test naming much at A levels, the question can give a complex name for an organic molecule and the student must be able to draw it out, but not vice-versa.
As a general rule (remember Chemistry, like real-life, must be intelligent and sensible, not dogmatic and meaningless), instead of memorizing priorities from your school lecture notes, just ask yourself, which functional group is more chemically reactive (ie. the most nucleophilic, electrophilic, lower Ea, etc), then such a functional group should be highlighted first in the name (because the most reactive functional group defines the molecule's most important properties), and hence give it priority. Do this because it makes intelligent sense to do so, not because your school lecture notes dogmatically say so.
PS. Just like how you (ie. everyone reading this) are advised to always be an intelligent, free-thinking individual who is self-responsible for what you choose to believe, be the captain of your own soul, instead of blindly following religious dogma of any religion.
-
Ultima, may i ask you how to determine if a (covalent) molecule can form favourable (ion-dipole) interactions with water ,hence soluble?
E.g https://www.dropbox.com/s/mb6a48ty02rgc7v/20160323_145224.jpg?dl=0
This is a covalent organic molecule, hence cannot use Energy of dissolution=Hydration enthalpy - lattice dissociation energy right?
Is it look at partial positive and partial negative?
Is it that, even if a molecule can form favourable (ion dipole) interactions with water, but not 100% always soluble?
-
Originally posted by Flying grenade:
Ultima, may i ask you how to determine if a (covalent) molecule can form favourable (ion-dipole) interactions with water ,hence soluble?
E.g https://www.dropbox.com/s/mb6a48ty02rgc7v/20160323_145224.jpg?dl=0
This is a covalent organic molecule, hence cannot use Energy of dissolution=Hydration enthalpy - lattice dissociation energy right?
Is it look at partial positive and partial negative?
Is it that, even if a molecule can form favourable (ion dipole) interactions with water, but not 100% always soluble?
Molecules are not ions, hence cannot have ion - permanent dipole interactions with protic polar water.For A level purposes, Solution enthalpy / entropy = Lattice dissociation enthalpy / entropy + Hydration enthalpy / entropy, is specifically for ionic species. (While it can theoretically be applied to covalent species, but due to the varying nature of different covalent species and their lack of a lattice structure under standard conditions, the relevant data becomes difficult to obtain and infeasible to apply with this Hess law derived formula, so instead direct experimental techniques are used instead).
Look at polarity and proticity. Molecule P is non-ionic (ie. no positive for negative formal charges present), aprotic, and relatively non-polar, hence is insoluble in water.
Yes, for instance, Cambridge can ask why amino acids are least water-soluble in their zwitterionic form at isoelectric point.
-
A protein has its lowest solubility at its isoelectric point. At isoelectric point, the amino acid carries no net electrical charge. Without a net charge, protein-protein interactions and precipitation are more likely, rather than to interact with water and undergo solvation.
Can get full marks?
-
Originally posted by Flying grenade:
A protein has its lowest solubility at its isoelectric point. At isoelectric point, the amino acid carries no net electrical charge. Without a net charge, protein-protein interactions and precipitation are more likely, rather than to interact with water and undergo solvation.
Can get full marks?
Zero marks. The website you quoted from failed to explain *why* precipitation rather than solvation (when the solvent is water, better to specify hydration) occurs at isoelectric point.I won't reveal the correct answer here, JC students can go ask your school teacher or private tutor. FlyingGrenade, no doubt you're gonna keep googling for the answer, but don't post the answer here and ruin the self-learning experience for others.
-
How to look for polarity and proticity?
How to know it's aprotic? Or how easy H+ is donated?
I thought that molecule is (relatively) polar?
Does it means non symmetrical doesn't affect polarity alot? Is the largest factor of affecting polar or not, the presence of lone pairs??
Molecule P has PdPd right? Because polar molecule arising from asymmetry?
If qn ask 'does a particular molecule dissolve in water'
Can we answer ' no pdpd attractions, cant form favourable interactions with water, hence insoluble' ?
I remember theres a rule of thumb 'like dissolves like' is this rule of thumb wrong?
Update : that molecule is weakly polar.
-
Originally posted by UltimaOnline:
Zero marks. The website you quoted from failed to explain *why* precipitation rather than solvation (when the solvent is water, better to specify hydration) occurs at isoelectric point.I won't reveal the correct answer here, JC students can go ask your school teacher or private tutor. FlyingGrenade, no doubt you're gonna keep googling for the answer, but don't post the answer here and ruin the self-learning experience for others.
Is it ionic bonding?
-
You're increasingly asking too many conceptual questions that you should be asking your school teacher or private tutor. It's appropriate to help you out on such questions during face-to-face tuition, but not on an online forum, which is meant for brief, light-touch guidance for students needing advice on specific exam-type (eg. TYS, prelim, tutorial, etc) questions, and not meant as a substitute for personal tuition (which is where your questions are headed). Join my tuition if you want such extensive conceptual guidance, or stick to asking brief queries on exam-type questions on this forum.
-
ClO-,ClO2-,ClO3-,ClO4-
-
Chlorate (I) ion, Chlorate (III) ion, Chlorate (V) ion, Chlorate (VII) ion
-
Hi Ultima , may i ask you,
Markovnikoff's rule states that in the addition of H-X or H-OH to a C=C bond in an asymmetrical alkene, the H atom attaches itself to a C atom that already holds the greater amount of Hydrogen atoms.
May i ask this : https://www.dropbox.com/s/9bahzdn6n96o91s/20160324_223727.jpg?dl=0
How to know Br and OH attach where??
For electrophilic addition of X2 (aq) to (asymmetrical) alkene to form halohydrin , how to determine X and OH form where?
-
Originally posted by Flying grenade:
Hi Ultima , may i ask you,
Markovnikoff's rule states that in the addition of H-X or H-OH to a C=C bond in an asymmetrical alkene, the H atom attaches itself to a C atom that already holds the greater amount of Hydrogen atoms.
May i ask this : https://www.dropbox.com/s/9bahzdn6n96o91s/20160324_223727.jpg?dl=0
How to know Br and OH attach where??
For electrophilic addition of X2 (aq) to (asymmetrical) alkene to form halohydrin , how to determine X and OH form where?
Cambridge will *not* accept Markovnikov's rule (or Zaitsev's rule, for that matter) as a reason, if Cambridge asks why a particular product is the major product (eg. "Draw the major product, and explain why it is the major product."). To get full marks, you need to draw both mechanisms (or at the very least, both the carbocation intermediates) for both products, then specify which carbocation is the more stable carbocation (ie. benzylic carbocation > allylic carbocation > tertiary carbocation > secondary carbocation > primary carbocation), then explain "The more stable the intermediate, the lower the Ea required, hence the faster the rate of reaction, hence we get more of the product, which we label as the major product."If you still can't figure it out, ask during tuition (after you join).
-
Since O is more electronegative than Br, is it O will form at the benzylic C atom ?
-
Originally posted by Flying grenade:
Since O is more electronegative than Br, is it O will form at the benzylic C atom ?
First of all, nothing "forms" at the benzylic C atom, wrong choice of words.Secondly, your reasoning is totally wrong (ie. it's not about electronegativity of O vs Br, because even if it was fluorine used instead of bromine, and F is of course more electronegative than O, the major and minor products discussed above will still be similar, albeit with *additional* possible beyond-H2-syllabus products due to the strongly oxidizing and highly reactive nature of fluorine).
Draw the full mechanisms to get both major & minor products (actually, there'll be a total of 3 possible products excluding stereochemistry when using aqueous halogen, can you figure out why?), and upload the image.
Note : many Singapore JCs teach wrongly that OH- attacks the carbocation, but it is actually H2O that is the competing nucleophile. Don't make this error in your mechanism.