Lurkers, register with SgForums and ask ur H2 Chem qns here!
-
repulsion : lone pair-lone pair > lone pair-bond pair > bond pair-bond pair
where does lone pair-lone electron , and bond pair-lone electron repulsion fit in to the above inequality?
-
Originally posted by Flying grenade:
repulsion : lone pair-lone pair > lone pair-bond pair > bond pair-bond pair
where does lone pair-lone electron , and bond pair-lone electron repulsion fit in to the above inequality?
Bear in mind that for alkyl radicals (ie. unpaired electron on a C atom), the unpaired electron occupies an unhybridized p orbital, orthogonal to the trigonal planar sp2 hybridized orbitals, while the three sp2 hybridized orbitals are used for sigma bond formations with adjacent C or H atoms.For other cases, you may take it that an unpaired single electron occupies less space and has less repulsion than either a lone pair or a bond pair.
This explains the 134 degrees bond angle of NO2 (in which the unpaired electron occupies a sp2 orbital, but has less repulsion with the bond pairs, hence bond angle increases slightly from 120 to 134 degrees; and the unhybridized p orbital is used for pi bonding with the an O atom in the resonance contributor, and thus having partial pi bonding with both O atoms in the resonance hybrid).
-
Another example of debye forces?
YJC/2016/JC2 block test
https://www.dropbox.com/s/lt8n85mfhkwn2ma/20160407_104726.jpg?dl=0
Cher say its pd-id forces
-
http://sgforums.com/forums/2297/topics/489346?page=20
Qn : bottom of page
-
Originally posted by Flying grenade:
Another example of debye forces?
YJC/2016/JC2 block test
https://www.dropbox.com/s/lt8n85mfhkwn2ma/20160407_104726.jpg?dl=0
Cher say its pd-id forces
All 3 types of van der Waals are present, but the predominant (ie. most extensive) van der Waals is permanent dipole - induced dipole Debye van der Waals forces. -
Originally posted by Flying grenade:
Hi Ultima, may i ask you,
in continuation to this https://www.dropbox.com/s/obq34agw4who7ay/forum%20ss.PNG?dl=0 (pg 19 of this forum thread) ,would ring strain affect geometric isomerism?
i've googled, and found these images, with 3 C atoms [http://images.tutorvista.com/cms/images/44/example-of--cis-trans-isomerism.png] ,
5 C atoms [http://wps.prenhall.com/wps/media/objects/340/348272/Instructor_Resources/Chapter_05/Text_Images/FG05_20-17UN.JPG] ,
and 6 C atoms [http://mcat-review.org/cis-trans-ring.gif]
, that can exhibit geometric isomerism, hence unsure how *big* ring is needed to exhibit geometric isomerism? ( in regard to your advice *This ring is too small to have geometric isomerism about the alkene group.* )
Rings can have geometric isomerism. Alkenes can have geometric isomerism. But alkenes as part of rings can have geometric isomerism only if the ring is large enough. Exactly how large? Try asking your school teacher or private tutor (for Singapore JC students reading this), see if he/she knows.Bonus : Why are trans alkenes usually more stable than cis alkenes? For alkenes as part of rings, are cis alkenes more stable or trans alkenes? How large (in terms of no. of atoms that make up the ring) must the ring be for cis alkenes to be as stable as trans alkenes? Below that number, which geometric isomer is more stable? Why? Above that number, which geometric isomer is more stable? Why? Try asking your school teacher or private tutor (for Singapore JC students reading this), see if he/she knows.
-
Originally posted by UltimaOnline:
Rings can have geometric isomerism. Alkenes can have geometric isomerism. But alkenes as part of rings can have geometric isomerism only if the ring is large enough. Exactly how large? Try asking your school teacher or private tutor (for Singapore JC students reading this), see if he/she knows.Bonus : Why are trans alkenes usually more stable than cis alkenes? For alkenes as part of rings, are cis alkenes more stable or trans alkenes? How large (in terms of no. of atoms that make up the ring) must the ring be for cis alkenes to be as stable as trans alkenes? Below that number, which geometric isomer is more stable? Why? Above that number, which geometric isomer is more stable? Why? Try asking your school teacher or private tutor (for Singapore JC students reading this), see if he/she knows.
Thank you for your reply.
Yeah, I'm unsure too, saw from the google images 3C atoms to 6 C atoms can all exhibit geometric isomerism ):
I agree "how large" is a very tough question
-
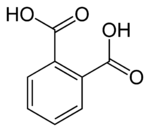
http://imgur.com/W5NBnst
^ In the above question, what will be formed for compounds 1 and 2 since both have a ring attached to a benzene rather than a hydrocarbon chain... will it be 1,2 dibenzoic acid for both 1 and 2??
-
Originally posted by Light5:
http://imgur.com/W5NBnst
^ In the above question, what will be formed for compounds 1 and 2 since both have a ring attached to a benzene rather than a hydrocarbon chain... will it be 1,2 dibenzoic acid for both 1 and 2??
Yes that's correct, both will yield benzene-1,2-dicarboxylic acid, aka phthalic acidhttps://en.wikipedia.org/wiki/Phthalic_acid.
-
Hey, Ultima, could you please post a list of all the steric and electronic factors that the we can be examined upon since sterics and electronics are 2 general areas and there are quite a few concepts in each of them that can get tested..now, im not asking you to list down every single one of those concepts and neither am i asking you to explain each and every one of them; i simply request you to list the concepts that you feel that can be asked in the CIE or H2 exams. I would also be very grateful if you could divide these factors into sub-headings like for eg : factors affecting reactivity of a compound, factors affecting feasibility of a reaction, factors affecting existence of a particular compound,etc. Once again, you do not need to explain those factors, ill search on them by themselves but if you could compile a list that comes into your head and post it here, i would be very grateful ....Thank You!!
-
Originally posted by Light5:
Hey, Ultima, could you please post a list of all the steric and electronic factors that the we can be examined upon since sterics and electronics are 2 general areas and there are quite a few concepts in each of them that can get tested..now, im not asking you to list down every single one of those concepts and neither am i asking you to explain each and every one of them; i simply request you to list the concepts that you feel that can be asked in the CIE or H2 exams. I would also be very grateful if you could divide these factors into sub-headings like for eg : factors affecting reactivity of a compound, factors affecting feasibility of a reaction, factors affecting existence of a particular compound,etc. Once again, you do not need to explain those factors, ill search on them by themselves but if you could compile a list that comes into your head and post it here, i would be very grateful ....Thank You!!
No can do, sorry, any 'list' for A level Chemistry would be more efficiently garnered through websites such as Jim Clark's website, or for Singapore students, OwlCove.Sg.The best way for me to help, is by giving guiding comments on specific exam questions you may come across. And that's what I'll do, no more, no less.
-
from wiki : Hydrobromic acid has a pKa of −9, making it a stronger acid than hydrochloric acid, but not as strong as hydroiodic acid. Hydrobromic acid is one of the strongest mineral acids known.
why hydroiodic acid stronger than hydrobromic acid stronger than HCl?
e.g. 1 mol of HBr vs 1 mol of HCl, both are strong acids, so dissociate completely to give 1 mol of H+ (or H3O+) correct?
why some acids that donate 1 mol of H+, some acids are stronger?
for HI and HBr, is it because H+ more readily dissociated ? but both strong acids dissociate completely ? so idk
-
Can odor be an indicator of chemical tests ?
for e.g. for the nucleophilic substition of acid chloride to amide, the product is HBr which is acrid smell
update ^ just noticed white fumes of HBr can also be observed,
what can be indicator of chemical tests? only color ?
-
.
-
.
-
. post more questions another time
-
Originally posted by Flying grenade:
from wiki : Hydrobromic acid has a pKa of −9, making it a stronger acid than hydrochloric acid, but not as strong as hydroiodic acid. Hydrobromic acid is one of the strongest mineral acids known.
why hydroiodic acid stronger than hydrobromic acid stronger than HCl?
e.g. 1 mol of HBr vs 1 mol of HCl, both are strong acids, so dissociate completely to give 1 mol of H+ (or H3O+) correct?
why some acids that donate 1 mol of H+, some acids are stronger?
for HI and HBr, is it because H+ more readily dissociated ? but both strong acids dissociate completely ? so idk
"Completely" actually means "almost completely". The Ka of HI(aq) > HBr(aq) > HCl(aq). But since HCl (the weakest of the 3 acids) is already 99.999% dissociated, at A levels we simplify matters by rounding it off to 100% and say that HCl is a "strong acid", and hence for aqueous (ie. water) solvent, you can say HCl, HBr and HI are all "strong acids" and "dissociate completely".Nonetheless at the same time, based on what you've learnt at A levels about acidic strength, you must still be able to also state that based on H-X bond dissociation enthalpy and stability of X- conjugate base, HI is still considered a stronger Bronsted-Lowry acid than HBr, which is still stronger than HCl, in terms of position of equilibrium and thermodynamic favorability.
So for A level purposes, state and explain both perspectives (ie. they're all strong acids which dissociate completely, but HI is still stronger than HBr which is still stronger than HCl). The distinction of acidic strengths becomes more pronounced and significant when using other solvents instead of water (ie. beyond the A level syllabus).
-
Originally posted by Flying grenade:
Can odor be an indicator of chemical tests ?
for e.g. for the nucleophilic substition of acid chloride to amide, the product is HBr which is acrid smell
update ^ just noticed white fumes of HBr can also be observed,
what can be indicator of chemical tests? only color ?
No, not as a main indicator, but only as a supportive indicator. So if a certain smell is expected or observed, go ahead and include it, but to get all the marks you still need other more objective observations. Color is accepted, but only if there are no other more objective observations. For many tests, more than 1 observation is expected simultaneously. So give all expected observations to secure the marks allocated. -
thank you !
F.R.S cannot occur at benzene because it's electrons are delocalised by resonance along the benzene ring and hence forming a pi electron cloud, hence forming strong bonds between the C atoms? can help check proper phrasing, thanks !
heat(not only u.v.?) can also initiate the breaking of cl2 molecule to form cl radicals?
-
my school has a organic chemistry reagents and conditions , and distinguishing/observation test quiz.
a screenshot provided :https://www.dropbox.com/s/kl3gjmpfyp9twlj/sss.PNG?dl=0
how is cold kmno4 be able to test presence of alkenes?
is it because only alkenes can react undergo mild oxidation with cold kmno4, and purple kmno4 is decolorised?https://www.dropbox.com/s/ge44xkvdzqt37kf/ssss.PNG?dl=0
both H2SO4 and NaOH can be used with KMnO4 ?
what's the diff by using AlCl3 and FeCl3? the similarity is it both have empty orbitals?
-
Originally posted by Flying grenade:
thank you !
F.R.S cannot occur at benzene because it's electrons are delocalised by resonance along the benzene ring and hence forming a pi electron cloud, hence forming strong bonds between the C atoms? can help check proper phrasing, thanks !
heat(not only u.v.?) can also initiate the breaking of cl2 molecule to form cl radicals?
First of all, don't use acronyms. Secondly, who told you this?"F.R.S cannot occur at benzene because it's electrons are delocalised by resonance along the benzene ring and hence forming a pi electron cloud, hence forming strong bonds between the C atoms."
That is not a correct explanation of why free radical substitution occurs on the side-chain of benzene rather than along the benzene ring itself. I don't want to spoil the fun for you guys, go ask your school teacher or private tutor why, see if he/she knows.
Yes, heating instead of UV light can also initial the presence of free radicals. However, your syllabus wants to test you to see if you know that UV light can accomplish this, and is more appropriate under certain industrial conditions (ie. where for various reasons you do not want high temperatures at this stage of the reaction), so you must still write "UV light" for the conditions in the A level exams, to generate free radicals.
-
im unsure why free radical substitution cannot(or can?) occur at benzene ring?
e.g. benzene ring needs to undergo electrophilic substitution with Cl2, anhydrous FeCl3 to form halogenoarene
-
Originally posted by Flying grenade:
my school has a organic chemistry reagents and conditions , and distinguishing/observation test quiz.
a screenshot provided :https://www.dropbox.com/s/kl3gjmpfyp9twlj/sss.PNG?dl=0
how is cold kmno4 be able to test presence of alkenes?
is it because only alkenes can react undergo mild oxidation with cold kmno4, and purple kmno4 is decolorised?https://www.dropbox.com/s/ge44xkvdzqt37kf/ssss.PNG?dl=0
both H2SO4 and NaOH can be used with KMnO4 ?
what's the diff by using AlCl3 and FeCl3? the similarity is it both have empty orbitals?
Yes, correct.Yes, correct. Temperature is the most crucial factor, not pH.
Yes, correct, plus the fact that both just need to accept 1 more (dative) bond from 1 more halide ion (from a halogen molecule) to achieve a stable octet, which is why it has high propensity to do so, consequently generating a unipositive formal charged X+ halonium electrophile, that may be readily attacked by the weak nucleophile benzene ring.
-
Originally posted by Flying grenade:
im unsure why free radical substitution cannot (or can?) occur at benzene ring?
e.g. benzene ring needs to undergo electrophilic substitution with Cl2, anhydrous FeCl3 to form halogenoarene
Go ask your school teacher or private tutor. Too much to explain over the forum. -
thanks !
then why need h2so4 or naoh with kmno4? why cannot kmno4 by itself or kmno4 with water?
