Lurkers, register with SgForums and ask ur H2 Chem qns here!
-
All aminos acid are polar right ? since alpha carbon is singly bonded to 4 diff groups of atoms
including glycine too, which has 2 H atoms bonded to alpha carbon
please kindly check page 36 of this thread where i uploaded my drawing of proline !
-
Originally posted by Flying grenade:
ultima can help check if i circle the correct R (in this case the alkyl ) group correctly, in my sketch of Proline, please !
https://www.dropbox.com/s/73pbse5uds7ib0j/20160705_181640-1.jpg?dl=0
Thank u !
More or less correct. But you may be penalized by Cambridge, because your circle (can be distorted circle, doesn't have to be perfectly circular) includes the alpha C atom, which is not part of the R group. -
Originally posted by Flying grenade:
All aminos acid are polar right ? since alpha carbon is singly bonded to 4 diff groups of atoms
including glycine too, which has 2 H atoms bonded to alpha carbon
please kindly check page 36 of this thread where i uploaded my drawing of proline !
Amino acids are not just polar, depending on pH and specific amino acid, they can even be dinegatively anionic, uninegatively anionic, zwitterionic, unipositively cationic, or dipositively cationic.All of these species (including the zwitterion) is even more 'polar' than a 'polar molecule' (the term implies overall neutral, and usually with no formal charges present), due to formal charges present and (other than the zwitterion) hence also ionic charges present.
-
Originally posted by UltimaOnline:
More or less correct. But you may be penalized by Cambridge, because your circle (can be distorted circle, doesn't have to be perfectly circular) includes the alpha C atom, which is not part of the R group.Noted, thank you so much! won't circle the alpha C atom in the future !
-
Thank you, Ultima !!!

-
[Not very important]
page 81 cs toh advanced guide
he said pH2O can be obtained from the Data booklet (given as a function of temp)
how to calculate it?
tried to figure out myself for some time but couldn't figure out. tried to research online and use the data booklet and use ideal gas eqn but to no avail.
from the internet, can obtain vapor pressure of(pure) H2O to be 3.17kpa (to 3sf) , at 25°C, consistent with example 3 of cs toh advanced guide page 81
Not sure how to calculate pH2O(g) as a function of temperature from data booklet as suggested by cs toh,
but it can be calculated by this method(not very simple, but manageable) :
peruse this
https://en.m.wikipedia.org/wiki/Vapour_pressure_of_water
and this
https://en.m.wikipedia.org/wiki/Antoine_equation
-
page 82 cstoh advanced guide, btm of page , elicited my thought on this :
my sch taught me this (when drawing structures )
double bond more favourable to form compared to single bond,
if can form covalent single bonds, it's preferable/more likely to form compared to dative bond
electronegative atom unlikely to donate lp of e- to form dative bond ( donate dative bond )
is this a good guideline?
-
what is the name of the reaction that causes displacement of the salt? Displacement reaction? Re-precipitation reaction?
for e.g. , SO42- + BaCl2 -> BaSO4 + 2Cl-
-
Originally posted by Flying grenade:
what is the name of the reaction that causes displacement of the salt? Displacement reaction? Re-precipitation reaction?
for e.g. , SO42- + BaCl2 -> BaSO4 + 2Cl-
Ionic precipitation.No comments regarding your other posts. To one, irrelevant to A level syllabus, don't waste your time. If you're itching to do some calculation involving water, calculating the pKa of H2O (at 25 degrees Celsius) is more relevant to the syllabus. Try calculating it out yourself before you google the answer. (Hint : you'll first need to calculate the molarity of pure water, before you can calculate the pKa of water).
To the other, you need to hands-on practice drawing and test yourself on as many different (and unusual) structures as possible, to test out for yourself your school guidelines to see if they're useful for yourself or not.
As practice (all you JC students, school teachers and private tutors reading this), try drawing out the full displayed structural formula for the following species, showing all lone pairs, bond pairs, formal charges, oxidation states, and dative bonds (if any), for the following structures. Challenge yourself to draw them out yourself first, before you google out the answer to check.
ClO
ClO2
Cl2O
Br2O3
Br2O4
Br2O5
Cl2O6
Cl2O7
S2O4 2-
S2O5 2-
S2O6 2-
H4P2O7
H5P3O10
KHF2
[ClF6]+
[AsF6]-
[XeF]+
[SbF6]-
[XeO2F]+
[XeOF3]+
[C6F5Xe]+
[(C6F5)2BF2]-
HN3 (not NH3)
[N5]+
IH5O6
[H2I2O10] 4-
[I2O9] 4-
((Methyl)2SiO3)3
C3H3N3O3
P4O6
P4O7
P4O10
P4O6S4
[P3O9] 3-
[P3O10] 5-
[P4O12] 4-
[SiO4] 4-
[Si2O7] 6-
[Si6O18] 12-
B4Cl4
B9Cl9
B2H6
B4H10
B5H9
B5H11
[B6H6] 2-
[B12H12] 2-
[B2(O2)2(OH)4] 2-
[B5O6(OH)4]-
[B4O5(OH)4] 2- -
OMG THANKKK YOUU SO MUCH ULTIMAAA!!! especially the list of molecules Thanks
-
Originally posted by Flying grenade:
OMG THANKKK YOUU SO MUCH ULTIMAAA!!! especially the list of molecules Thanks
Uhhh you really gonna try to draw them all out? Don't force yourself too hard. A few of these are not meant to be able to be drawn out at A levels (was just being cheeky or guailan for some of the structures). Also, you can google out all the structures yourself. No need to post them here. And don't bother trying to ask me on the forum "why like this?!?" or "like that also can?!?". Go ask your school teacher or private tutor. Too inefficient to teach drawing via forum (especially the tougher structures not intended for A levels).Btw, you'll first need to calculate the molarity of pure water, before you can calculate the pKa of water at 25 degrees Celsius.
-
what does 'FA' abbreviation stand for in titration solutions e.g. FA1 and FA2?
always wanted to know abt this but couldn't find out
searched internet and legit cant find anything FA related to chemistry ,
My guess is Formal Analyte
xD
-
Originally posted by Flying grenade:
what does 'FA' abbreviation stand for in titration solutions e.g. FA1 and FA2?
always wanted to know abt this but couldn't find out
searched internet and legit cant find anything FA related to chemistry ,
My guess is Formal Analyte
xD
It stands for "Hao Siao" (Hokkien). No meaning lah! If Cambridge felt like it, they also can use CB1, CB2, except that the candidates may be distracted with all the giggling. -
why polymer, then cannot have geometric (cis-trans) isomerism? there's double bond within polymer
-
Originally posted by Flying grenade:
why polymer, then cannot have geometric (cis-trans) isomerism? there's double bond within polymer
In protein polypeptides, because the lone pair on the N atom is delocalized by resonance, hence the peptide bond in the resonance hybrid actually has partial double bond character, which means sigma bond rotation is restricted by the partial pi bond, which means that whichever geometric isomer (usually the more stable trans isomer) the protein polypeptide was originally generated with (ie. during the intra-cellular translation process involving ribosomes, mRNA and tRNA), that will be the permanent geometric isomer for that particular peptide bond in the protein polypeptide. -
THANKSS ULTIMAA !! thanks for all the knowledge
-
page261 cstoh advanced guide d orbital splitting
page 222 2nd print George chong inorganic chem book d orbital splitting
page 450 K.S. Chan physical inorganic chem book
george chong's and K.S. Chan's book show energy of d* orbitals are of higher energy than ground state 3d orbitals, in a free metal ion
cstoh's book shows the splitting causes some orbitals to be of less and more energy compared to 3d isolated orbitals
-
Originally posted by Flying grenade:
page261 cstoh advanced guide d orbital splitting
page 222 2nd print George chong inorganic chem book d orbital splitting
page 450 K.S. Chan physical inorganic chem book
george chong's and K.S. Chan's book show energy of d* orbitals are of higher energy than ground state 3d orbitals, in a free metal ion
cstoh's book shows the splitting causes some orbitals to be of less and more energy compared to 3d isolated orbitals
Here is your answer. Always cross reference across multiple sources, *plus* think critically for yourself : ask yourself which version should be more correct, and why? Apply yourself and your own free-will with self-respect and self-responsibility, instead of blindly following dogma (be it academic, family, cultural, societal, ethical, moral, religious, etc).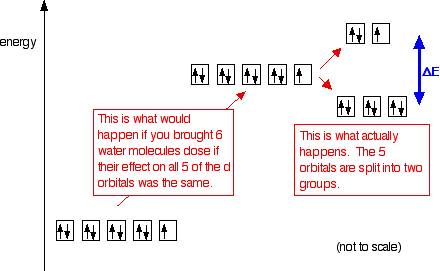
http://www.chemguide.co.uk/inorganic/complexions/colour.html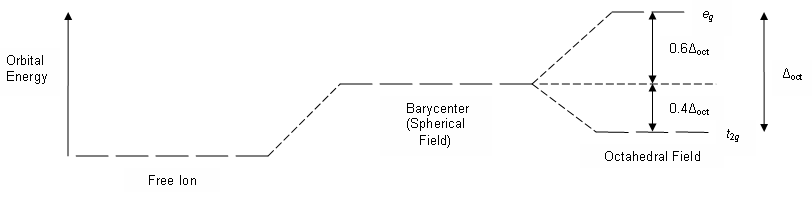
https://en.wikipedia.org/wiki/Crystal_field_theory#Crystal_field_stabilization_energy -
OMMMMMMMMGGGGGGGGGGG THANKSSS ULTIMAAAA
u are godly
-
version of chemguide and wiki is indeed better for this case
-
Hi, I have a question for chemistry and read you can post them here?
5.00g of an anhydrous Grp 2 metal nitrate loses 3.29g in mass when heated strongly
2M(NO3)2 => 2MO+4NO2+O2
What is metal M?
Magnesium
Calcium
strongtium
barium
-
Originally posted by Molestermannn69:
Hi, I have a question for chemistry and read you can post them here?
5.00g of an anhydrous Grp 2 metal nitrate loses 3.29g in mass when heated strongly
2M(NO3)2 => 2MO+4NO2+O2
What is metal M?
Magnesium
Calcium
strongtium
barium
I'll leave it to Flying Grenade (don't disappoint me) to show you how to solve it. -
cs toh didnt notice this ,or at least he didn't update this
pg 188 chem advanced guide
the 'slow' and 'fast' is wrong, and must be cancelled right?
-
Originally posted by Flying grenade:
cs toh didnt notice this ,or at least he didn't update this
pg 188 chem advanced guide
the 'slow' and 'fast' is wrong, and must be cancelled right?
Obviously. Now go solve Molestermannn69's qn. -
Originally posted by UltimaOnline :
I'll leave it to Flying Grenade (don't disappoint me) to show you how to solve it.
5.00g of an anhydrous Grp 2 metal nitrate loses 3.29g in mass when heated strongly
2M(NO3)2 (s) => 2MO(s) + 4NO2(g) + O2(g)
What is metal M?
Flying grenade's suggested solution :
Mass MO must be 5.00 - 3.29 = 1.71g
mol = mass/mr
let mr of M be x
ηM(NO3)2 = 5/(x+124)
ηMO=1.71/(x+16)
since stoichiometric ratio of M(NO3)2 to MO is 1:1
we can equate ηM(NO3)2 = ηMO
solving the equations , we obtain x=40.1 (3sf)
Metal M is Calcium.
