Lurkers, register with SgForums and ask ur H2 Chem qns here!
-
https://www.dropbox.com/s/xjzn8hn5arv6z7y/Screenshot_2016-08-30-18-29-18.png?dl=0
Enols, or more formally, alkenols, are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene(olefin) with a hydroxyl group attached to one end of the alkene double bond.
is it unstable, and may decompose? what will it decompose into?
other miscellaneous thoughts :
difficult to synthesise
molecule not stable
-
Originally posted by Flying grenade:
https://www.dropbox.com/s/xjzn8hn5arv6z7y/Screenshot_2016-08-30-18-29-18.png?dl=0
can this molecule exist?
is it unstable, and may decompose? what will it decompose into?
other miscellaneous thoughts
not industrially useful hence dont have molecule's name or name of the class of cpds
difficult to synthesise
molecule not stable
?
Enols exist in equilibrium with their prototropic tautomer, ketones / aldehydes. Draw the mechanism (either neutral pH or acid catalysed, up to you) to prototropically tautomerize ethenol to its carbonyl tautomer, ethanal.As a challenging A grade question (which I will not provide the answer here, go ask your school teacher or private tutor), Cambridge may ask you to explain where the position of equilibrium lies, and (more challengingly) exactly why.
-
I see, thank you ultima !!
-
Ethenol, vinyl alcohol
https://en.m.wikipedia.org/wiki/Vinyl_alcohol
-
MDA
https://en.m.wikipedia.org/wiki/Malondialdehyde
-
Originally posted by Flying grenade:
MDA
https://en.m.wikipedia.org/wiki/Malondialdehyde
A BedokFunland JC H2 Chemistry Challenge QuestionQ1. Why does the position of equilibrium lie on the side indicated?
Q2. In organic solvents, the cis-isomer is favored, whereas in water the trans-isomer predominates. Explain.
(I won't reveal the answers here and spoil the fun, you can go ask your school teacher or private tutor.)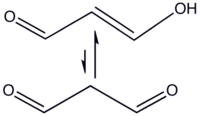
-
Hi I need your help again.
https://drive.google.com/open?id=0Bziu2m7eQFelZXZhZEdBd1Y2akE
For f, since deltaH = deltaH2 - deltaH3 + deltaH(H2O), am i correct to say that residual heat from experiment 1 (hcl+naoh) causes the temperature change for experiment 2 (mg+hcl) to be higher than expected, making the value more exothermic. Furthermore, heat lost to the surroundings (mgo+hcl) causes the temperature change for experiment 3 to be lower than expected, making the value less exothermic. I feel that my answers are contradicting themselves as there should be residual heat from both experiment 1 and 2 causing the enthalpy change for 3 to be incorrect.
For g, i have absolutely no clue on how to approach the question.
-
Originally posted by Nikkilyx:
Hi I need your help again.
For f, since deltaH = deltaH2 - deltaH3 + deltaH(H2O), am i correct to say that residual heat from experiment 1 (hcl+naoh) causes the temperature change for experiment 2 (mg+hcl) to be higher than expected, making the value more exothermic. Furthermore, heat lost to the surroundings (mgo+hcl) causes the temperature change for experiment 3 to be lower than expected, making the value less exothermic. I feel that my answers are contradicting themselves as there should be residual heat from both experiment 1 and 2 causing the enthalpy change for 3 to be incorrect.
For g, i have absolutely no clue on how to approach the question.
Can't see the image at all. Write out the URL of the image, no need to use html code to embed.If it's a TYS or Prelim qn, just state the source, eg. 2015 RJC Prelim P2 Q3.
-
Hi this is the link
https://drive.google.com/open?id=0Bziu2m7eQFelZXZhZEdBd1Y2akE
-
Originally posted by Nikkilyx:
Hi I need your help again.
https://drive.google.com/open?id=0Bziu2m7eQFelZXZhZEdBd1Y2akE
For f, since deltaH = deltaH2 - deltaH3 + deltaH(H2O), am i correct to say that residual heat from experiment 1 (hcl+naoh) causes the temperature change for experiment 2 (mg+hcl) to be higher than expected, making the value more exothermic. Furthermore, heat lost to the surroundings (mgo+hcl) causes the temperature change for experiment 3 to be lower than expected, making the value less exothermic. I feel that my answers are contradicting themselves as there should be residual heat from both experiment 1 and 2 causing the enthalpy change for 3 to be incorrect.
For g, i have absolutely no clue on how to approach the question.
f) The discrepancy is due to the differing state symbol of water. In the experiment, it's H2O(g). In the calculations, it's H2O(l).g) Since the enthalpy change of hydration applies equally for both alternative intermediate steps in our enthalpy cycle generating [Mg(H2O)n]2+(aq), they in effect cancel out (when applying Hess' Law) and hence does not affect our enthalpy change calculations of Mg to MgO.
-
for f, is there another possible reason? because the question states reasons...
-
Originally posted by Nikkilyx:
for f, is there another possible reason? because the question states reasons...
The other reasons are less important, and are due to experimental limitations, validity and reliability issues, such as the ones you've mentioned. -
If D.O.U. >4 , we suspect a b. ring, if dou <4 , confirm bo b ring present is it?
-
Originally posted by Flying grenade:
If D.O.U. >4 , we suspect a b. ring, if dou <4 , confirm bo b ring present is it?
Obviously. -
2015 A level p3 qn 1C
i calculated D.O.U. of C10H16O is 3. what can i do with this info?
thanks ultima
-
Originally posted by UltimaOnline:
Obviously.I SEE , TY ULTIMA for reply
-
Originally posted by Flying grenade:
A level p3 qn 1C
i calculated D.O.U. of C10H16 is 3. what can i do with this info?
thanks ultima
U coconaden. Can u spot what's wrong with what you wrote above? As for what u can do with it, what do u think? If u have no idea how to use d.o.u, then don't bother using it. Go ask ur school teacher or private tutor. -
D.O.U. of 1 double bond is 1 right
i calculated D.O.U. for the above molecule =3
but the molecule only have 2 double bond ! T-T help ultima
-
Originally posted by Flying grenade:
D.O.U. of 1 double bond is 1 right
i calculated D.O.U. for the above molecule =3
but the molecule only have 2 double bond ! T-T help ultima
U coconaden. First, spot what's wrong with ur original post. -
Originally posted by UltimaOnline:
U coconaden. First, spot what's wrong with ur original post.damn. sorry. ok corrected roflmao
-
spot the difference spot the mistake is fun
-
Originally posted by Flying grenade:
D.O.U. of 1 double bond is 1 right
i calculated D.O.U. for the above molecule =3
but the molecule only have 2 double bond ! T-T help ultima
Eh u (still a) coconaden lah! Ask yourself, why exactly does benzene ring have 4 d.o.u??? Or u dunno and just blindly memorized that fact?!? Once u can answer that qn, u can answer ur own qn, about why molecules A, B and C have 3 d.o.u each. -
eh
i worked out d.o.u. of benzene =4 .
D.O.U.(benzene) = (14-6)/2 =4
-
i worked ouuuuuuttttt for that molecular formula for A,B,C have 3 d.o.u. ,
my qn
is one double bond = 1 d.o.u.?
cos all of A,B,C have only 2 double bonds in their structural formula
how/in what way can use dou to write out deductions?
teach me plsss
-
i dont want write C and H have comparable number of H atoms or ratio C/H is approx one or not approx one
e.g. 16H , 10 C. is 1.6 approx one? is 1.4 approx one? is 1.2 approx one? is 14H atoms and 10 C atoms comparable in number?