2016 H2 Chemistry JC1 & 2 students post your questions here
-
Originally posted by Ng.keebin:
Why does pKa value of hydrohalic acids decrease down the group?
The strength of HX increases (ie. Ka increases or pKa decreases) down the halogens (Group VII or Group 17) for 2 main reasons : decreasing magnitudes of endothermic H-X proton dissociation enthalpies (quote relevant Data Booklet H-X bond enthalpies, and explain that the effectiveness of orbital overlap to form the H-X sigma bond decreases with increasing principal quantum number and the concordant increasing diffusiveness of electron orbitals), and increasing stabilities of X- halide ion conjugate base due to decreasing anionic charge densities in aqueous solutions. -
Originally posted by Ng.keebin:
Salicyclic acid contains both a hydroxyl and a carboxylic acid group. Why will the hydroxyl group bonded directly to the benzene ring not be substituted upon addition of SOCl2?
 Image : https://en.wikipedia.org/wiki/Salicylic_acid
Image : https://en.wikipedia.org/wiki/Salicylic_acid
Simple answer :
Because only aliphatic alcohols (and carboxylic acids) react with SOCl2 and PCl5, but not phenols.
Further answer :
Due to the sideways overlap of p-orbital of the O atom and the pi-orbital of the benzene ring, a lone pair on the O atom is delocalized by resonance to form a pi bond with the sp2 C atom of the benzene ring, giving the C-O bond in the resonance hybrid partial double bond character, which is thus strong and does not cleave readily. Hence all such nucleophilic substitutions on the benzene ring (which involves the potential leaving group having partial double bond character with the benzene ring in the resonance hybrid) are strongly resisted.
Even deeper further answer :
In addition, depending on the solvent used (eg. nucleophilic or not) and any other reagents present (eg. pyridine in SOCl2 reaction), let us consider the following 6 nucleophilic substitution mechanisms for the nucleophilic substitution reaction between phenol and SOCl2 or PCl5 : SN1, SN2, SNi (nucleophilic substitution with internal return), and Nucleophilic Aromatic Substitutions via either SNAr (addition-elimination) or via the benzyne intermediate (elimination-addition) or via SRN1 (free radical nucleophilic aromatic substitution).
SN2 isn't possible for phenols because the electrophilic C atom is itself part of the benzene ring, which hence poses an insurmountable steric hindrance (ie. it is geometrically, sterically and electrostatically impossible for the electron-rich Cl- nucleophile to approach the electrophilic C atom from within the electron-rich benzene ring itself as required by the SN2 mechanism) and concordantly, generating a geometrically unstable and thermodynamically infeasible pentavalent transition state (bear in mind this is nucleophilic substitution, not nucleophilic addition).
SN1 and SNi for arenes are highly thermodynamically unfavorable because upon attempted elimination of the leaving group (ie. the addition product of the phenolic group with the SOCl2 or PCl5 reagent), the positive formal charge on the consequent (highly unstable and thus thermodynamically infeasible to form) aryl carbocation species is highly destabilizing for 2 reasons : the positive formal charge would be on a sp hybridized C atom (ie. high % of s orbital character results in increased electronegativity which exacerbates the destabilizing effect of a positive formal charge), and furthermore the positive formal charge can *not* be delocalized by resonance (which is only possible for benzylic carbocations and carbanions, ie. when the formal charge is on the C atom outside, not within, the benzene ring), as the ring strain due to angle strain (ie. destablization due to significant deviation from the ideal bond angles of the sp hybridized C atom as predicted by VSEPR theory) prevents formation of an allene resonance contributor.
Nucleophilic aromatic substitutions do not readily occur and have high activation energy barriers (do you know why? Cambridge may ask you to suggest reasons for this). SNAr (addition-elimination) mechanism requires (preferably 2 or 3) strongly electron-withdrawing by both induction and resonance groups (preferably NO2 nitro groups) at the ortho and para positions (in salicylic acid, the ortho COOH group is indeed electron-withdrawing by induction and resonance, but not as strongly as NO2 groups because a formal positive charge on the N atom is more strongly electron-withdrawing by induction compared to a mere partial positive charge on the C atom).
The benzyne intermediate (elimination-addition) mechanism requires a very strong base for deprotonation of benzene (usually employing the amide NH2- ion, in which case the final product would be phenylamine rather than chlorobenzene) and the benzyne intermediate is itself highly unstable due to reasons discussed earlier (ie. destabilizing ring strain caused by angle strain due to significant deviation from the ideal bond angles about a sp hybridized C atom as predicted by VSEPR theory resulting in an unusually weak triple bond and hence being thermodynamically unfavorable), with equally unstable cumulene and biradical resonance contributors.
SRN1 (free radical nucleophilic aromatic substitution) requires a free radical initiator, and for which halogens are the leaving group to be eliminated rather than the incoming nucleophile. -
Further reading for those of you who might be interested in the stereochemistry of the SOCl2 (thionyl chloride) mechanism :
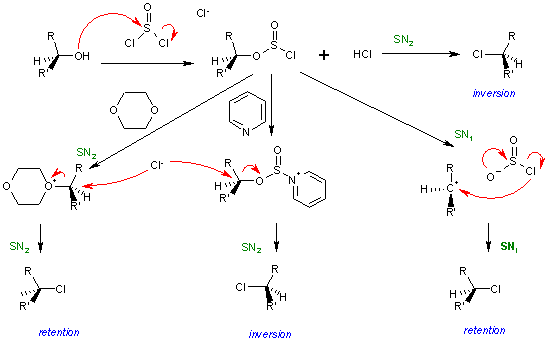
Image source : https://en.wikipedia.org/wiki/SNi
If you have difficulty understanding the diagram above, James Ashenhurst explains the reaction in detail here : http://www.masterorganicchemistry.com/2014/02/10/socl2-and-the-sni-mechanism/ -
Reminder : this thread is open to ALL Singapore JC 1 and JC 2 students studying H1 and H2 Chemistry, whether you're from RJC or MI, whether you already have your own private tutor or have no tuition at all. You just need to register an SgForums account (it's free!), and you get to enjoy the benefit of getting high quality, trustworthy and reliable help with your Chemistry questions free-of-charge.
-
1. What happens when CH3CO(CH2)2CH=C(CH3)2 reacts with HBR?
2. A certain industrial cleaner and paint solvent was distilled to produce a single compound D. When D reacted with 2,4-DNPH, an orange ppt was produced. With alkaline aq I2, D gave a pale yellow ppt. D did not react either with warm acidifed KmnO4 or with aq Br2. Reduction of D with H2 over a catalyst produced equimolar mixture of 2 isomers E and F with molecular formula C4H10O.
Is D an aldehyde or ketone? Reduction of D with H2 over a catalyst shows that it is an aldehyde (my notes said that ketone cannot be reduced by H2) but no reaction with acidified KmnO4 shows that it is a ketone.
-
Originally posted by Ephemeral:
1. What happens when CH3CO(CH2)2CH=C(CH3)2 reacts with HBR?
2. A certain industrial cleaner and paint solvent was distilled to produce a single compound D. When D reacted with 2,4-DNPH, an orange ppt was produced. With alkaline aq I2, D gave a pale yellow ppt. D did not react either with warm acidifed KmnO4 or with aq Br2. Reduction of D with H2 over a catalyst produced equimolar mixture of 2 isomers E and F with molecular formula C4H10O.
Is D an aldehyde or ketone? Reduction of D with H2 over a catalyst shows that it is an aldehyde (my notes said that ketone cannot be reduced by H2) but no reaction with acidified KmnO4 shows that it is a ketone.
Q1. Electrophilic addition of HX unto the nucleophilic alkene occurs. As for determining the major product, Cambridge will *not* accept "because of Markovnikov's rule" as an explanation. You need to specify "Tertiary carbocations are more stable than secondary carbocations ; the more stable the intermediate, the lower the Ea required, hence the faster the rate of reaction, generating more of the product, which we label as the major product".
As far as the H2 syllabus is concerned, carbonyl compounds do not react with hydrogen halides or hydrohalic acids, as the X- nucleophile has lower anionic charge densities and thus less nucleophilic, Bronsted-Lowry basic and Lewis basic, compared to say, the cyanide ion (written as CN-, but when drawing out the mechanism, Cambridge requires you to clearly indicate the uninegative formal charge on the C atom and not the N atom, or you'll be penalized), as C is not electronegative enough to stabilize a negative formal charge (the only reason why CN- can even exist as an intermediate is due to the high % s orbital character of the sp C in CN-).
Beyond the H2 syllabus, the addition of HX to carbonyl compounds does occur, but the position of equilibrium lies mostly to the carbonyl side, as determined thermodynamically by favourable entropy change. This is similar to the position of equilibrium lying significantly more towards the carbonyl side than the geminol diol side, but even moreso.
Q2. -__-" Don't trust your school notes. Gaseous hydrogen (with nickel or platinum catalyst) does indeed reduce aldehydes, ketones and even nitriles, but not carboxylic acids, esters and amides. -
What is the type of reaction in the second step when using Grignard reagent?
Why does the second step occur and why does it not stay as -- O^-MgX^+?
-
Originally posted by Ephemeral:
What is the type of reaction in the second step when using Grignard reagent?
Why does the second step occur and why does it not stay as -- O^-MgX^+?

Source : https://en.wikipedia.org/wiki/Grignard_reaction
Bronsted-Lowry acid-base protonation or proton transfer reaction. In anhydrous solvents, it does stay as O- ionically bonded to +MgX (with some degree of covalent character). But because you (ie. whoever carries out the reaction) want to obtain the organic alcohol product (with a longer carbon chain), thus you would subsequently add water (the least toxic and cheapest Bronsted-Lowry acid available) to protonate the alkoxide (which is significantly Bronsted-Lowry basic because O- is relatively unstable due to relatively high anionic charge density as the negative formal charge is on the small O atom with small atomic radius being a period 2 element) to obtain your desired (conjugate acid) alcohol product now with a longer carbon chain (as Grignard reagents are carbon nucleophiles which undergo nucleophilic addition), together with (MgX)OH as a by-product (not side-product, don't confuse the two). -
If benzene ring contains delocalised electrons, why can't it conduct electricity?
-
Originally posted by Ng.keebin:
If benzene ring contains delocalised electrons, why can't it conduct electricity?
Because unlike the giant covalent lattice structures of graphene (which stack together in layers to form graphite), benzene consists of simple, discrete covalent molecules, and hence can only delocalize electrons within a single small molecule of benzene (ie. electrons cannot jump from one benzene molecule to the next, since the molecules are separate or discrete), while in graphene (being a giant covalent lattice of benzene rings joined together, so to speak, see image below), the delocalized electrons can travel across the entire giant covalent lattice of the graphene, which is the medium that conducts the electricity (ie. flow of electrons).
Image source : https://en.wikipedia.org/wiki/Graphene -
Hello UltimaOnline,
I have some questions at hand:
Q1:
With reference to the second and fourth row in the diagram, what’s the mathematical basis behind the linearising of the first and second order concentration against time graphs? In other words, why do I get a linear graph when I ln the concentration in a first order reaction and when I reciprocate the concentration in a second order reaction?
Q2: ACJC 13/P1/Q5
I find the phrasing of the choices confusing. If I accept the answer as D, can I take “the phosphorus atoms of both oxides” to mean some of the phosphorus atoms of both oxides? (Although this will also make C and B acceptable choices too.)
Alternatively, if I take “the phosphorus atoms of both oxides” to mean all of the phosphorus atoms of both oxides, there would have been no correct answer, given that there is a trigonal pyramidal P at P4O9?
Q3: If I mix tetrachloromethane with methanol, what are the intermolecular forces of attraction formed (debye forces?) and destroyed (presumably id-id and H bond). Debye forces are weaker than pd-pd forces but how does that account for no heat being evolved? Likewise, when I mix trichloromethane (pd-pd) with propanone (pd-pd), why would heat be evolved?
Thank you! :)
-
Originally posted by gohby:
Hello UltimaOnline,
I have some questions at hand:
Q1:
With reference to the second and fourth row in the diagram, what’s the mathematical basis behind the linearising of the first and second order concentration against time graphs? In other words, why do I get a linear graph when I ln the concentration in a first order reaction and when I reciprocate the concentration in a second order reaction?
Q2: ACJC 13/P1/Q5
I find the phrasing of the choices confusing. If I accept the answer as D, can I take “the phosphorus atoms of both oxides� to mean some of the phosphorus atoms of both oxides? (Although this will also make C and B acceptable choices too.)
Alternatively, if I take “the phosphorus atoms of both oxides� to mean all of the phosphorus atoms of both oxides, there would have been no correct answer, given that there is a trigonal pyramidal P at P4O9?
Q3: If I mix tetrachloromethane with methanol, what are the intermolecular forces of attraction formed (debye forces?) and destroyed (presumably id-id and H bond). Debye forces are weaker than pd-pd forces but how does that account for no heat being evolved? Likewise, when I mix trichloromethane (pd-pd) with propanone (pd-pd), why would heat be evolved?
Thank you! :)
Hi Gohby,
Q1. The linearizing of molarity-against-time graphs to determine 2nd and 3rd orders by use of ln and reciprocal functions are beyond the H2 syllabus, and thus not required for students to know. Cambridge will give relevant guidance if these functions are to be used. Nonetheless, it's a useful bonus to teach your students about using these, and will still be acceptable by Cambridge (though they'll usually structure their questions such that the student will need to do other within-syllabus procedures and checks that will already be sufficient to determine the order of reaction, so this is just an extra bonus).
To address your question more directly is in the realm of mathematics rather than chemistry, and thus if you would like to pursue a deeper explanation, you could start another thread requesting the assistance of the resident mathematics experts Eagle and WeeWS. The key to understanding the linearity of the molarity-against-time graphs after the ln and reciprocal functions are applied, are deriving the Integrated (ie. applying the Integration function of Calculus unto the rate laws) 1st and 2nd Order Rate Laws. Once these are derived using Calculus, then the graphical linearity becomes a natural and mathematically obvious expression of the Integrated Rate Laws. For further reading, see links below.
https://en.wikipedia.org/wiki/Rate_equation
http://www.chm.davidson.edu/vce/kinetics/integratedratelaws.html
http://chemwiki.ucdavis.edu/Core/Physical_Chemistry/Kinetics/Methods_of_Determining_Reaction_Order/Using_Graphs_to_Determine_Rate_Laws
One last point though, is that for the H2 syllabus, Cambridge will instead use another graphical linearizing method to hint to the student that the reaction is of 2nd order kinetics with respect to a particular reactant. Graphical linearizing can be achieved by squaring (ie. power raised to 2nd order) the molarity or partial pressure of the reactant, and plotting against initial rate (with x and y axes interchangeable, after all it's a linear graph). This is to test A level students to see if they are able to correctly interpret the linear graph to mean that the rate of reaction is *directly proportional* (hence linear) to the square (ie. raised to the power of 2) of the molarity or partial pressure of the reactant, and thus deduce the order of reaction is 2nd order with respect to that reactant. Specifically, see Singapore A levels 2010 P2 Q3 for such an example.
Q2. The question's fault for not being specific. In which case you have to consider all the options together and see which interpretation makes the most sense (here, it is ALL atoms rather than SOME atoms). So D is still the best answer. The "tetrahedral arrangement" in option D refers to the electron geometry rather than molecular geometry. Most Singapore JC school teachers (and private tutors) unwisely teach their students 'shape' or 'geometry' to directly mean 'molecular geometry' or 'ionic geometry', when the correct pedagogical approach would be to teach students to first work out the 'electron geometry', and thereafter derive the concordant 'molecular geometry' or 'ionic geometry'.
On a related note, most Singapore JC school teachers (and private tutors) also unwisely teach students to identify orbital hybridization (sp, sp2, sp3, etc) in terms of single vs double vs triple bonds, when the correct pedagogical approach would be to teach orbital hybridization in terms of electron geometries. In other words, do H2 Chem students truly understand the *purpose* and *nature* of orbital hybridization (ie. in relation to VSEPR theory, sigma & pi bonds, and lone pair residence), or do they blindly apply the 'single vs double vs triple bonds' rules of their school lecture notes without understanding?
An effective way to test your students on their understanding on this, is to ask them to identify orbital hybridizations of non-carbon atoms in a variety of molecules, and going beyond just sp, sp2 and sp3 orbital hybridizations. For more able students, you can bring in resonance as well, and how it relates to and/or affects orbital hybridizations and vice-versa.
Q4. Tetrachloromethane with methanol : since H bonding exists between methanol, and only van der Waals (a mixture of all 3 : Keesom or pd-pd, Debye or pd-id and London Dispersion or id-id) forces exist between tetrachloromethane with methanol, thus newer intermolecular attractions are weaker than original intermolecular attractions, and thus the solvation or mixing will be endothermic.
Trichloromethane with propanone : slightly trickier, since both are polar aprotic molecules. In such a case, at A levels, students are not expected to state for certain (ie. without experimental data) whether the mixing will be exothermic or not, but A level students are only required to state that the reaction *may* or *could* be exothermic, because the newly formed intermolecular attractions *may* or *could* be slightly stronger than original intermolecular attractions (which for MCQs this understanding will usually suffice to determine the answer, especially when combined with elimination MCQ strategy).
Not all van der Waals (ie. whether we're talking about Keesom or pd-pd, Debye or pd-id and London Dispersion or id-id) forces are equal in strength, before and after mixing of 2 species. The newly formed (Keesom or Debye or even London Dispersion) van der Waals forces could be weaker or stronger than the original van der Waals forces (even when considering the same specific type of van der Waals forces, eg. Keesom forces).
Why? Consider the molecular geometries and dipoles present : trichloromethane (with longer C-Cl bonds and a shorter C-H bond ; the Cl atoms are strongly delta negative while the C atom is strongly delta positive) is tetrahedral while propanone is trigonal planar (with C being strongly delta positive and O being strongly delta negative due to both induction and resonance).
Hence, the newly formed molecular interactions (mainly and most significantly Keesom forces, but all 3 types of van der Waals forces are of course present) are stronger, because the geometries and dipoles allow it : the strongly delta negative O of propanone forms strong Keesom attractions with the strongly delta positive C (with H posing negligible steric hindrance) of trichloromethane, while the strongly delta negative Cl atoms of propanone forms strong Keesom attractions with the strongly delta positive C of propanone.
A level students are strongly advised to draw out both molecules to illustrate (and annotate) these intermolecular Keesom van der Waals forces, even if the Cambridge question doesn't specifically require so. This is because Cambridge will award marks as long as relevant content is written by the student, regardless of whether textual or graphical. -
Updated (Thurs) : I've just edited the preceding post and included additional content. For those of you who read the earlier version of the preceding post yesterday (Wed), you might like to revisit it.
-
hello
-
Here's a Chemistry Calculation Challenge for all of you 2016 Chemistry students (both JC1 & JC2, both H1 & H2).
Approximately 7.98 g of an unknown metal oxide reacts with 3.667 dm3 of carbon monoxide to generate carbon dioxide and the solid metal (at conditions where the molar volume of a gas may be taken to be 24.45 dm3). 125.0 cm3 of 1.60 mol/dm3 of HCl (aq) was required to completely convert all of the solid metal into its chloride salt. Using mathematical calculations and chemical reasoning, identify the metal.
Warning : You may *not* assume the oxidation state of the metal is necessarily the same in both its oxide and its chloride ; ie. the OS of the metal may or may not be the same, eg. V2O5 --> V --> VCl3.
After attempting the above question, you may post your final answer (ie. identity of metal) here and I'll tell you if your answer is correct, but do not post the complete solution here (don't ruin the fun for others). -
Question: What is the mass of 300 cm^3 of sulfur dioxide measured at stp?
My working: 300cm^3 = 0.3dm^3
No. of mol = 0.3/22.7 = 0.01321
Therefore mass = 0.01321*22.7 = 0.3g
Do I need to do all those working to get the final answer which is given in the qn but in dm^3 form or is my answer wrong? And if it is correct, then why is the mass the same as the volume(?) given in the qn?
-
Originally posted by acorel:
Question: What is the mass of 300 cm^3 of sulfur dioxide measured at stp?
My working: 300cm^3 = 0.3dm^3
No. of mol = 0.3/22.7 = 0.01321
Therefore mass = 0.01321*22.7 = 0.3g
Do I need to do all those working to get the final answer which is given in the qn but in dm^3 form or is my answer wrong? And if it is correct, then why is the mass the same as the volume(?) given in the qn?
Your molar volume of gas at stp and your molar mass of SO2 are both wrong. -
Hi ultima, one of your post(that i lost the link i couldn't find) you mentioned about that one cannot assume that initial [H+] approx the concentration at eqm, because initial [H+] is too great. And a quadratic equation arise. But we can indeed use graphic calculator or scientific calculator to solve quadratic equations as they are approved for use in exams
-
Originally posted by Flying grenade:
Hi ultima, one of your post(that i lost the link i couldn't find) you mentioned about that one cannot assume that initial [H+] approx the concentration at eqm, because initial [H+] is too great. And a quadratic equation arise. But we can indeed use graphic calculator or scientific calculator to solve quadratic equations as they are approved for use in exams
I didn't say you're not allowed to, I just said you didn't have to. In other words, for A levels, Cambridge will set the questions such that the initial molarity is >> than the change in molarity, such that the equilibrium molarity can always be approximated to initial molarity, since Cambridge won't force you to use graphing calculator for H2 Chem. (But if your school exam papers use Uni level Chem questions, as Singapore JCs often do, such that the initial molarity is not >> than the change in molarity, then for such questions, you're not allowed to approximate equilibrium molarity back to initial molarity, and you *must* use graphing calculator to solve the quadratic equation which arises).
There is one type of A level exam question which is the exception though, that still doesn't require you to use graphing calculator, but does require you *not* to approximate equilibrium molarity back to initial molarity. Such a question was raised by Gohby a few weeks ago, and another such question was asked before by Cambridge (for Singapore A levels) over a decade ago.
If the pH at equilibrium (eg. at initial, at equivalence point, etc) is given to you (either stated in the question or graphically), then change in molarity of H+ (or in some questions OH-) can be thus derived (ignore negligible contribution by the self-ionization of water), which means there's no need to solve any quadratic equation and hence no need for graphing calculator, in which to calculate the equilibrium molarity (or in Gohby's question, the initial molarity) of the weak acid (or in some questions weak base), you have to take the initial molarity, minus away the known (ie. non-algebraic) change in molarity to dissociate to generate H+ (or in some questions base hydrolysis to generate OH-), to get an accurate value for molarity of the weak acid (or in some questions weak base) at equilibrium (or in Gohby's question at initial), in order to (as was the case in the Cambridge Singapore A level question a decade ago) calculate Ka or pKa (or in some questions Kb or pKb) as required by the question. -
Nov 2002/P3/Q8 Or
-
Originally posted by Fxwhy:
Nov 2002/P3/Q8 Or
Eh specify exactly what it is about the qn or solution u dunno or wanna clarify leh.
Also, 2002 too old liao, I dun have the TYS qn. Can u take a photo and upload the qn to ur Facebook or Twitter, and link the image here? -
Hello UltimaOnline,
I have some questions which I would like to clarify:
- HCI/08/P1/Q4
For option B, both hydrazine and hydrogen peroxide form hydrogen bonds and dispersion forces. What makes the bp of hydrazine lower? Also, I was thinking that D could be the answer because the δ- on N is weaker than the δ- on O, thus it would be a weaker base.
2. RVHS/13/P1/Q32
For option 1, I can accept that magnesium has a melting point greater than 97.8. However, without memorising the actual melting point of magnesium, how would I know that its melting point is below 1083? Also, could you explain why option 2 is factually correct (only the electron for copper in the 4s subshell is delocalised right), and that such information can be deduced from the table?
3. NJC/13/P1/Q31
How can we be sure the enthalpy changes in 1 & 2 cannot be determined experimentally?
Thank you! :)
-
Originally posted by gohby:
Hello UltimaOnline,
I have some questions which I would like to clarify:
- HCI/08/P1/Q4
For option B, both hydrazine and hydrogen peroxide form hydrogen bonds and dispersion forces. What makes the bp of hydrazine lower? Also, I was thinking that D could be the answer because the δ- on N is weaker than the δ- on O, thus it would be a weaker base.
2. RVHS/13/P1/Q32
For option 1, I can accept that magnesium has a melting point greater than 97.8. However, without memorising the actual melting point of magnesium, how would I know that its melting point is below 1083? Also, could you explain why option 2 is factually correct (only the electron for copper in the 4s subshell is delocalised right), and that such information can be deduced from the table?
3. NJC/13/P1/Q31
How can we be sure the enthalpy changes in 1 & 2 cannot be determined experimentally?
Thank you! :)
Yo Gohby,
Q1. Yes, because N is less electronegative than O.
Q2. Lousy qn. From the given data alone, all 3 options cannot be deduced. From data given + Data Booklet / Periodic Table (which is thus implied), options 1 & 2 can be deduced to be *probable*. 3 is wrong because it's too generalized, and Chemistry (being a microcosm of real life) always has exceptions to every rule. You can only deduce the melting point of Mg is *probably* somewhere in between, as (by using Data Booklet) the cationic charge density is also somewhere in between. For metallic bonding, the d electrons also participates, though to a much lesser extent than the s electrons.
Q3. Because in adding water, you can't guarantee every water molecule added will be used for hydration instead of for solution. If you say do backwards and carry out dehydration instead (a more controllable process), that's applying Hess Law already *evil laughter from Hess*. C and H2 do not spontaneously (ie. delta G = +ve) form methane. You need to couple it with other thermodynamically favourable processes (eg. biochemical or industrial) to form methane through a series of steps. See https://en.wikipedia.org/wiki/Methane#Production -
Originally posted by UltimaOnline:
Yo Gohby,
Q1. Yes, because N is less electronegative than O.
Q2. Lousy qn. From the given data alone, all 3 options cannot be deduced. From data given + Data Booklet / Periodic Table (which is thus implied), options 1 & 2 can be deduced to be *probable*. 3 is wrong because it's too generalized, and Chemistry (being a microcosm of real life) always has exceptions to every rule. You can only deduce the melting point of Mg is *probably* somewhere in between, as (by using Data Booklet) the cationic charge density is also somewhere in between. For metallic bonding, the d electrons also participates, though to a much lesser extent than the s electrons.
Q3. Because in adding water, you can't guarantee every water molecule added will be used for hydration instead of for solution. If you say do backwards and carry out dehydration instead (a more controllable process), that's applying Hess Law already *evil laughter from Hess*. C and H2 do not spontaneously (ie. delta G = +ve) form methane. You need to couple it with other thermodynamically favourable processes (eg. biochemical or industrial) to form methane through a series of steps. See https://en.wikipedia.org/wiki/Methane#ProductionHi UltimaOnline,
Am I right to say that the bp of hydrazine is lower than that of hydrogen peroxide because of stronger pd-pd AND H-bonds (even though they both form 2 H bonds/molecule) because O is more electronegative than N, thus the aforementioned forces formed will experience stronger attraction?
RVHS/13/P1/Q13
I agree with A being the "best" answer, but is C an incorrect answer, given that magnesium chloride is acidic and magnesium oxide is basic?
-
Originally posted by gohby:
Hi UltimaOnline,
Am I right to say that the bp of hydrazine is lower than that of hydrogen peroxide because of stronger pd-pd AND H-bonds (even though they both form 2 H bonds/molecule) because O is more electronegative than N, thus the aforementioned forces formed will experience stronger attraction?
RVHS/13/P1/Q13
I agree with A being the "best" answer, but is C an incorrect answer, given that magnesium chloride is acidic and magnesium oxide is basic?
Yes, though focus on the H bonding, not the van der Waals.
Option C is wrong, because MgO while basic, isn't sufficiently soluble (due to highly endothermic lattice dissociation enthalpy) to generate a strong alkali (ie. high pH). Even option A is incomplete or partially incorrect, as aqueous alkalis at room temperature cannot dissolve SiO2 (due to an even higher endothermic lattice dissociation enthalpy, in turn due to SiO2 being a giant covalent lattice structure). Molten (not aqueous) alkalis, or (at the very least) concentrated alkalis at high temperatures, are required.