2016 H2 Chemistry JC1 & 2 students post your questions here
-
h2 chemistry A levels 2007 Paper 2
Qn 4, C (ii)
suggest the size of the cl-c-cl bond, my answer is 115deg but the answer from tys is 120deg
Qn 4, d (i)
for stage ll, my answer is decomposition but tys answer is elimination
-
Originally posted by Shinyphua:
h2 chemistry A levels 2007 Paper 2
Qn 4, C (ii)
suggest the size of the cl-c-cl bond, my answer is 115deg but the answer from tys is 120deg
Qn 4, d (i)
for stage ll, my answer is decomposition but tys answer is elimination
It's obviously trigonal planar, 120 degrees. Think of it as a ketone, with the 2 alkyl groups replaced by Cl atoms.Best answer is to combine both, ie. decomposition via elimination. When in doubt as to which answer Cambridge wants, write both answers. As long as your answers don't contradict, Cambridge will credit the required answer, and ignore the other answer. BFJC ftw.
-
https://twitter.com/doublehalfarrow/status/746629255522443264 How does A undergo intramolecular nuclophilic substitution between its amine and alkyl halide to form D?
-
Explain why gaseous ethanoic acid, Ch3COOH has an apparent molecular mass of 120 instead of 60
Ans: this is because the the gas particles consist of hydrogen bonded dimers
Is it because organic acid in gaseous state have molecular mass twice of what it is expected??? If yes, why so??? So what if it consist of hydrogen bonded dimers?
-
https://twitter.com/doublehalfarrow/status/746634870940565504 Why is [OH-] divided by 2 in the answer?
-
Originally posted by Fxwhy:
https://twitter.com/doublehalfarrow/status/746629255522443264 How does A undergo intramolecular nuclophilic substitution between its amine and alkyl halide to form D?
What do you mean, "how does it undergo intramolecular nuclophilic substitution?". It undergoes because of the mechanism, that's how (by definition)!If you mean "how do you know" then it's because the no. of C atoms in A and D are the same, and the degree of unsaturation increases by 1 (due to formation of cyclic ring), that's how you know.
-
Originally posted by Christabellw:
Explain why gaseous ethanoic acid, Ch3COOH has an apparent molecular mass of 120 instead of 60
Ans: this is because the the gas particles consist of hydrogen bonded dimers
Is it because organic acid in gaseous state have molecular mass twice of what it is expected??? If yes, why so??? So what if it consist of hydrogen bonded dimers?
Not for all organic acids, but especially for small carboxylic acids. And not just gaseous state, but in the liquid state as well, and in non-polar solvents as well.For such questions, you're required to draw (and label!) the following hydrogen-bonded dimer :
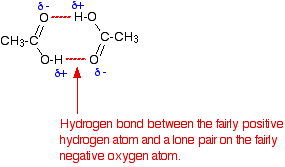
http://www.chemguide.co.uk/organicprops/acids/background.html
-
Originally posted by nicolemantou:
https://twitter.com/doublehalfarrow/status/7466…
Why is [OH-] divided by 2 in the answer?
Because adding equal volumes of both reactant solutions, doubles the volumes, hence halves the molarities of the aqueous ions. -
For the bond angle of cl-c-cl bond of cocl2, even with the presence of the double bond with O, the bond angle won't be affected and remain at 120 degrees?
-
h2 chemistry A Level TYS
2007 Paper 3 qns 2(e)ii: My answer for the reaction I is base hydrolysis but the ans is neutralisation. Why is it neutralisation?
2007 Paper 3 qns 3(d)i: How do the product CH3CHO form from the reaction?
2007 Paper 3 qns 3(d)ii: Can CH2O decolourise purple KMnO4 and liberate CO2 gas? Cos i thought that ketones can't do so...
-
Originally posted by ACA-Milkshake:
For the bond angle of cl-c-cl bond of cocl2, even with the presence of the double bond with O, the bond angle won't be affected and remain at 120 degrees?
For A level purposes, the expected answer remains at 120 degrees, ie. Cambridge will award full credit (of 1 mark) for 120 degrees. In actuality, or at Uni levels, the bond angle in phosgene is 112 degrees, but Cambridge won't require this value for A levels.
https://en.wikipedia.org/wiki/PhosgeneAt most, Cambridge may ask, "Suggest a reason for the slight deviation from the 120 degrees as predicted by VSEPR theory", to which there are several contributing reasons (beyond the A level syllabus), one of which is as you mentioned (the greater repulsion of the double-bond).
If the A level candidate wishes to incorporate such contributing factors that result in a deviation in the basic bond angles as predicted by VSEPR theory, the candidate (if he is exam-smart and wishes to safeguard his marks) must do so with a written explanation, first giving the basic bond angle of 120 degrees (with brief explanation), then make mention of factors which cause deviation, then suggest a concordantly modified bond angle.
-
Originally posted by ACA-Milkshake:
h2 chemistry A Level TYS
2007 Paper 3 qns 2(e)ii: My answer for the reaction I is base hydrolysis but the ans is neutralisation. Why is it neutralisation?
2007 Paper 3 qns 3(d)i: How do the product CH3CHO form from the reaction?
2007 Paper 3 qns 3(d)ii: Can CH2O decolourise purple KMnO4 and liberate CO2 gas? Cos i thought that ketones can't do so...
The amide group isn't hydrolyzed in the reaction, rather the N-H group is being deprotonated, ie. removal of H+, leaving behind a uninegative formal charged N atom as illustrated in the question.The mechanism is significantly beyond A levels. For A level purposes, use simple pattern recognition and mathematics. Based on the original reaction equation given, and based on the new reactants of ethanoate and methanoate ions in a 1:1 ratio, let A = H atom, B = methyl group, we mathematically obtain carbonyl molecules with AA, AB, BA and BB groups, hence we obtain a 1:2:1 ratio of methanal, ethanal and propanone. Notice that Cambridge isn't asking for an explanation, but merely "suggest the products formed and the ratio they're obtained".
KMnO4 oxidizes methanal to methanoic acid to carbonic(IV) acid, which decomposes into CO2(g) + H2O(l), with thermodynamically favorable positive entropy change (since CO2(g) is gaseous), and as predicted by Le Chatelier's principle, since CO2(g) leaves the reaction solution, pulling the position of equilibrium over to the RHS.
-
Why is the C-O-H bond slightly larger than the C-S-H bond angle?
For this question, the answer given is that O and S contains 2 lone pairs and 2 bond pairs, as O is more electronegative than S, the bond pair of electrons are held more closely towards O, hence, a stronger repulsion.
My question is, can i answer in terms of the size of the molecule? O is smaller than S, and thus the C and H atoms are of closer proximity to the molecule than when its a large molecule like S, therefore, the lone pair - bond pair repulsion is of greater magnitude.
-
Originally posted by Shinyphua:
Why is the C-O-H bond slightly larger than the C-S-H bond angle?
For this question, the answer given is that O and S contains 2 lone pairs and 2 bond pairs, as O is more electronegative than S, the bond pair of electrons are held more closely towards O, hence, a stronger repulsion.
My question is, can i answer in terms of the size of the molecule? O is smaller than S, and thus the C and H atoms are of closer proximity to the molecule than when its a large molecule like S, therefore, the lone pair - bond pair repulsion is of greater magnitude.
Singapore JCs often use electronegativity to explain the deviation from the basic bond angles of period 2 elements as predicted by VSEPR theory, when discussing period 3 elements. Other than cases involving electronegative F (eg. why NF3 bond angles deviate from NH3 bond angles), using electronegativity to explain the deviation is actually incorrect, or rather, only a secondary factor (Chemistry, being a microcosm of real-life, is multi-factorial).The more important reason, is due to the significantly lesser extent of orbital hybridization (which requires energy, and thus only thermodynamically justified for less diffused valence orbitals of period 2 elements), due to the significantly lesser extent of electron-pair repulsions, due to the significantly more diffused valence orbitals (which the lone pairs reside in, or the bond pairs are formed from the overlap of) of period 3 elements.
Consequently, the atoms of period 3 elements use their mostly unhybridized orbitals for their lone pairs to reside in, and for overlapping head-on or end-on to form sigma bonds. In the case of C-S-H, the S atom uses it's mostly unhybridized p orbitals to overlap head-on or end-on to form its sigma bonds with the C and H atoms, as well as for 1 of the lone pairs to reside in, with the remaining lone pair residing in the mostly unhybridized s orbital. This results in the C-S-H bond angle (with 2 orthogonally oriented p orbitals used for head-on or end-on overlap with the C and H atoms) being close to the unhybridized p orbital bond angle of 90 degrees.
So you're partially right that it's about the size, though you'll need to elaborate (as I did) to secure your marks. Your school (and Singapore JCs in general) aren't entirely wrong, it's just that electronegativity isn't the most important contributing factor here (unlike in cases such as NF3 vs NH3).
However, all this discussion may be moot, because as far as A levels are concerned, Cambridge won't require the student to give any answer other than the basic bond angles as predicted by VSEPR theory for period 2 elements, ie. just state 104.5 degrees as if the S atom were an O atom, and include the usual explanation about the S atom having 2 lone pairs and 2 bond pairs, and that lone pair - lone pair repulsions > lone pair - bond pair repulsions > bond pair - bond pair repulsions, that would gain you full marks.
After writing the basic answer above, if you like, if you have the time, or if (unexpectedly, though I'd see it as a pleasant surprise) Cambridge specifically asks you to explain why the C-S-H bond angle deviates from the C-O-H bond angle, then you can elaborate further to why the C-S-H bond angle is actually less than the C-O-H bond angle, giving either my (more correct) explanation, or your school's (less correct) explanation, or both. But again, because asking this would be beyond the A level syllabus, it is rather unlikely for Cambridge to ask why the C-S-H bond angle deviates from the C-O-H bond angle for the actual A level exam (ie. your question, which you presumably took from your school's mid-year revision package).
-
h2 A Level TYS
2008 Paper 3 Qns 2e(iii): Why does the amount of H from alkane D times 2? And from the ratio between the moles of C and H, the molecular formula i got is C2H5 instead of C4H10 and i am not sure why.
2008 Paper 3 Qns 2f: How do they know that the R'CO2 in the reactants, is A?
2009 Paper 3 Qns 3c(iii): In the answer booklet, the answer for the amount of Na2O = 1/2 x [(9.00x10^-3) - (4.00x10^-3)]. My question is where did they get the 4.00x10^-3 from?
-
Originally posted by ACA-Milkshake:
h2 A Level TYS
2008 Paper 3 Qns 2e(iii): Why does the amount of H from alkane D times 2? And from the ratio between the moles of C and H, the molecular formula i got is C2H5 instead of C4H10 and i am not sure why.
2008 Paper 3 Qns 2f: How do they know that the R'CO2 in the reactants, is A?
2009 Paper 3 Qns 3c(iii): In the answer booklet, the answer for the amount of Na2O = 1/2 x [(9.00x10^-3) - (4.00x10^-3)]. My question is where did they get the 4.00x10^-3 from?
2008 Paper 3 Qns 2e(iii) :Because each mol of H2O contains 2 mol of H (from alkane D). The mole ratio gives you empirical formula of C2H5, which gives a wrong (and non-valid non-integer) degree of unsaturation for an alkane, which should be 0 degrees. Hence multiply by 2 (and if it doesn't work, try 3) to obtain the molecular formula of C4H10 (with the correct degree of unsaturation).
2008 Paper 3 Qns 2f :
Because from previous parts of the question, you've already found that A is C3H7COOH, C is C5H12, D is C4H10, which means that C must be R-R' (ie. 2 + 3 = 5), hence if we let R be 2 carbons and R' be 3 carbons, then D (4 carbons) would be R-R, then E must be R'-R' which has 6 carbons (eg. hexane or an isomer of), which also means A must be R'COOH (which is deprotonated to be used as R'COO-), which means B must be RCOOH, which must therefore be C2H5COOH.
2009 Paper 3 Qns 3c(iii) :
From the acid-base titration, you know the total mol of OH- from both sodium oxide and sodium peroxide. From the redox titration, based on the mol of MnO4- required, you know the mol of hydrogen peroxide oxidized, and thus by stoichiometry, you also know the mol of sodium peroxide present and the mol of OH- from the hydrolysis of the sodium peroxide.
Hence, you can calculate the mol of OH- generated from sodium oxide alone (ie. total mol of OH- which is 9.00x10^-3, minus away mol of OH- from peroxide which is 4.00x10^-3, which will give you mol of OH- from oxide, which is 5.00x10^-3).
Divide this value by 2 (since each mol of sodium peroxide generates by hydrolysis 2 mol of NaOH), to obtain the final answer of 2.50x10^-3 mol of sodium oxide.
-
2009 TYS A Level Paper 3 Qns 4d(ii): In the answer, why will the 2 carbons that are bonded to the Br atoms eventually combine and bond to NH after undergoing nucleophilic subs? I thought they would form 2 NH2 groups, one on each of the 2 Carbons?
-
Originally posted by ACA-Milkshake:
2009 TYS A Level Paper 3 Qns 4d(ii): In the answer, why will the 2 carbons that are bonded to the Br atoms eventually combine and bond to NH after undergoing nucleophilic subs? I thought they would form 2 NH2 groups, one on each of the 2 Carbons?
Always draw out (if you're unable to visualize) the mechanism (for either forward and/or backward reaction), that'll lead you to the correct structure and answer. In this particular question, the forward reaction is more thermodynamically feasible than the backward reaction, but for the sake of elucidating J, we'll imagine the backward mechanism here.Br- attacks C, NRH gets kicked out as NRH-, gets protonated as NRH2. Another Br- attacks the other C (of the R group), NH2 gets kicked out as NH2-, gets protonated as NH3. What's left behind is J, which includes the 2 Br atoms.
-
https://mobile.twitter.com/itsliyingg/status/750022746466234368
Part g and h
-
Originally posted by Liying98:
https://mobile.twitter.com/itsliyingg/status/750022746466234368
Part g and h
In an octahedral complex, the dz2 and dx2-y2 orbitals have a higher energy level compared to the dxy, dyz and dxz orbitals.Since n = 4 (ie. 4 unpaired electrons) is calculated from the formula given, and since Cr2+ is [Ar] 4s0 3d4, it means that all 4 electrons of the Cr2+ ion are unpaired, which in turn means the Cr2+ ion in this particular octahedral hexaaquachromium(II) coordination complex must be high spin (ie. small magnitude of energy gap between d-d* orbitals). This is consistent with H2O being a (relatively) weak field ligand.
On the other hand, if the 6 ligands were stronger field ligands, eg. cyanide CN- ligands in the octahedral hexacyanochromate(II) coordination complex, then you would expect to calculate n = 2 (ie. 2 unpaired electrons), which means that all 4 electrons of the Cr2+ ion must occupy the 3 lower energy dxy, dyz and dxz orbitals, which in turn means the Cr2+ ion in this particular octahedral hexacyanochromate(II) coordination complex must be low spin (ie. large magnitude of energy gap between d-d* orbitals).
To show the electron distribution in the transition metal within the coordination complex as required by the MJC question, check out the low spin vs high spin electron distributions for the Fe3+ (ie. [Ar] 4s0 3d5) example on this Wikipedia page, and modify it for Cr2+ (ie. [Ar] 4s0 3d4).
https://en.wikipedia.org/wiki/Spin_states_(d_electrons)
Due to inter-electron electrostatic repulsions, it is both kinetically and thermodynamically less favorable to add a negatively charged electron onto a negatively charged anionic coordination complex such as hexacyanochromate(III), and therefore you can expect the standard reduction potential to be significantly less positive, or concordantly more negative ; compared to a when adding a negatively charged electron onto a positively charged cationic coordination complex with the same transition metal ion with the same oxidation state such as hexaaquachromium(III). -

-
Originally posted by UltimaOnline:

formula for x axis : [metal]/[ligand]
interpretation : stoichiometry and hence coordination complex formula can deduce thereafter
-
Originally posted by Flying grenade:
formula for x axis : [metal]/[ligand]
interpretation : self explanatory
Cambridge could use this question as a challenging (ie. A grade) A level H2 Chem exam question. Your submitted answers would score : zero marks and zero marks. -
formula for x axis : volume/cm3
or [metal ion]/[ligand]
take for example , from the graph, the point of most intense colored solution , which is the point of maximum absorbance, is approx 0.75cm3
thereafter, find mol ratio ion : ligand by mol(ion)/mol(ligand)
and hence can deduce molecular formula of the complex
info im missing is.. which particular ion and ligand, so i can construct the exact Chemical Equation needed
need the chemical eqn to solve this, which in turn need the identity of the particular ion and ligand, right?
-
Originally posted by Flying grenade:
formula for x axis : volume/cm3
or [metal ion]/[ligand]
take for example , from the graph, the point of most intense colored solution , which is the point of maximum absorbance, is approx 0.75cm3
thereafter, find mol ratio ion : ligand by mol(ion)/mol(ligand)
and hence can deduce molecular formula of the complex
info im missing is.. which particular ion and ligand, so i can construct the exact Chemical Equation needed
need the chemical eqn to solve this, which in turn need the identity of the particular ion and ligand, right?
Still zero marks. Your formula is wrong. From the graphical data given, you need to be able to ascertain the exact formula of the coordination complex.Use M to represent the metal ion, and L to represent each ligand. Write the formula of the coordination complex in terms of M and L, and leave the ionic charge of the coordination complex as an unknown n-.